Comparing protein expression in Sf9 and Sf21 insect cells
This case study shows how we used mCherry Red Fluorescent Protein to compare recombinant protein expression in Sf9 versus Sf21 insect cells.
One of the well-established eukaryotic expression systems used at Peak Proteins for expressing secreted, intracellular and membrane proteins is the flashBACTM baculovirus insect cell system (licensed from Oxford Expression Technologies (OET). We routinely use Sf9 and Sf21 cultured insect cells isolated from Spodoptera frugiperda (Fall Armyworm). Different clients often express a preference for one cell line over the other and we wanted a better understanding of which cell host is likely to be preferable. Fluorescent proteins, including the monomeric bright red fluorescent protein mCherry derived from dSRed of Discosoma sea anemones, are a powerful tool for cell biology. We utilised a 6-His tagged mCherry construct to undertake a simple time course in Sf9 and Sf21 cells.
Transfection and Expression of protein in insect cells
Sf9 cells were used to directly generate intracellular mCherry recombinant P0 virus. In this study, either Sf21 cells or Sf9 cells at a point of infection cell density of 2.5x106cells/ml were then infected with this recombinant baculovirus to produce the mCherry red fluorescent protein. Samples were taken at 24 hr, 48 hr, 72 hr and 96 hr post-infection.
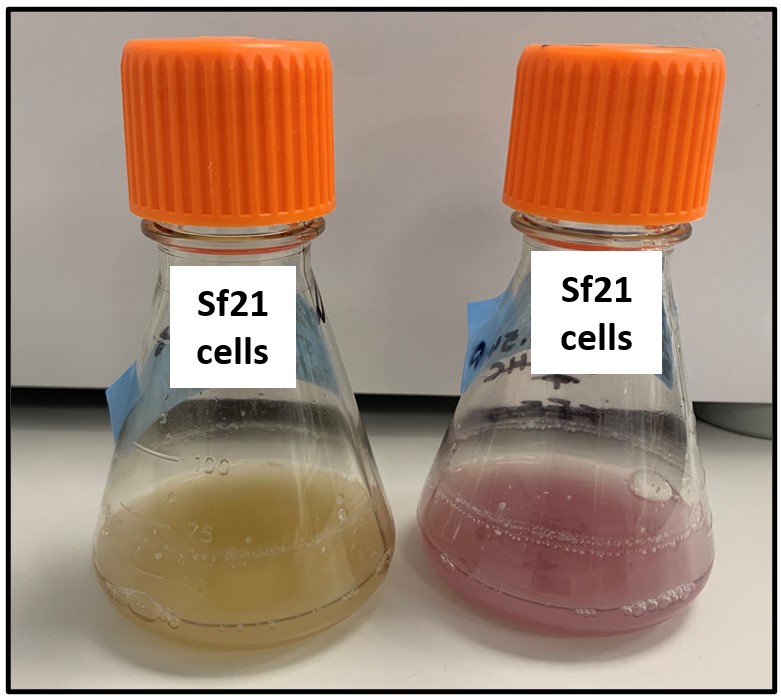
Figure 1: Uninfected control on the left. Flask infected with mCherry baculovirus on the right.
Protein purification from insect cells using Nickel affinity.
Sf21 cells infected with recombinant 6His tagged mCherry baculovirus were harvested 48 hours post infection. Cells were spun down and protein was purified from the pellet using Nickel-NTA affinity chromatography. ES-MS/MS peptide-mapping confirmed the presence of mCherry.
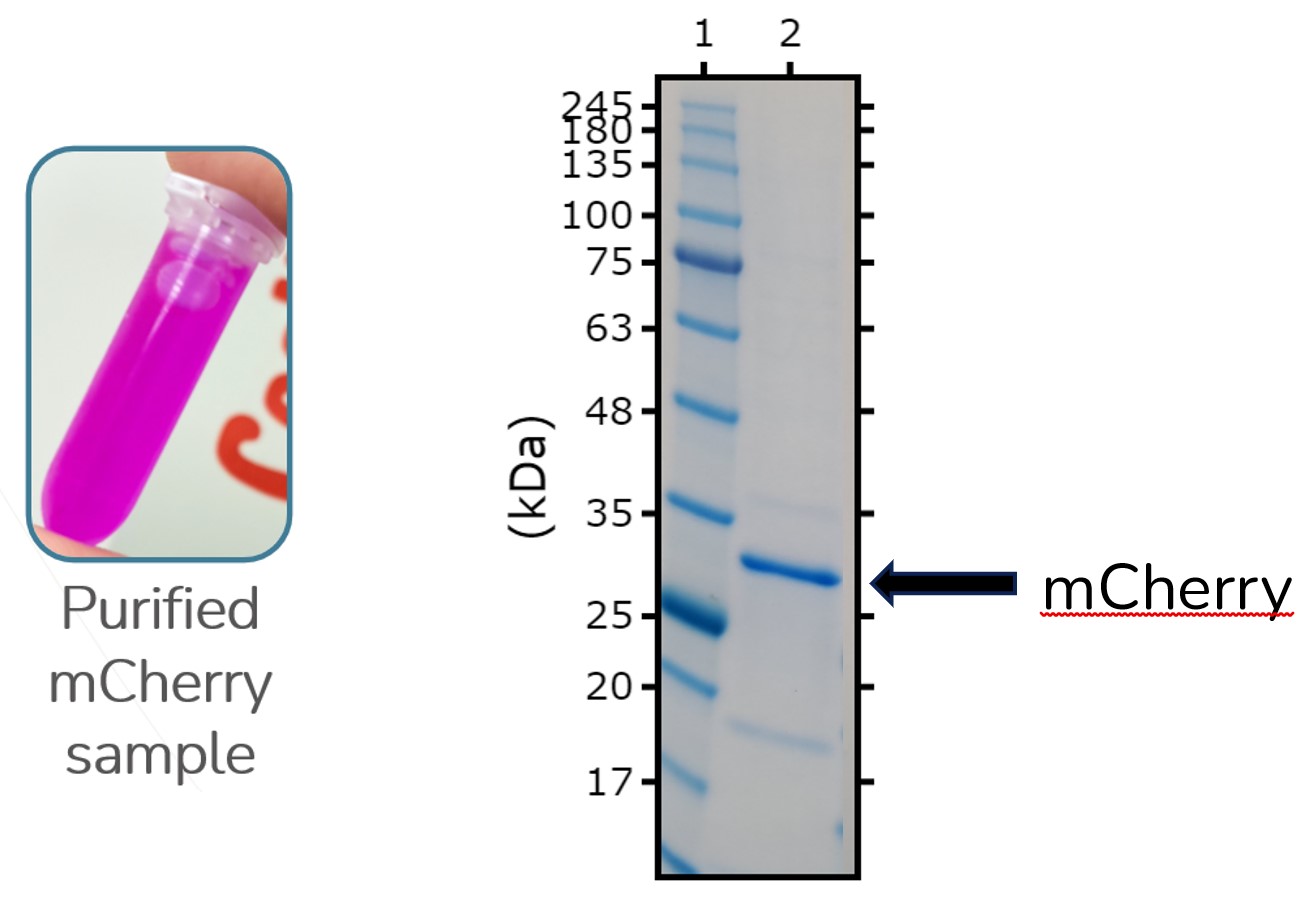
Figure 2: Vial with purified mCherry sample in along with an SDS PAGE of the same sample.
Quantification of recombinant mCherry Protein Expression from Sf9 vs Sf21
Purified mCherry was used to create a calibration curve of known concentrations using a 96-well fluorescence microplate reader. Samples taken from Sf9 and Sf21 cells infected with mCherry baculovirus were read on a fluorescence microplate reader. The calibration curve was used to identify the concentration of intracellular mCherry protein expression in each sample taking into account the cell viability.
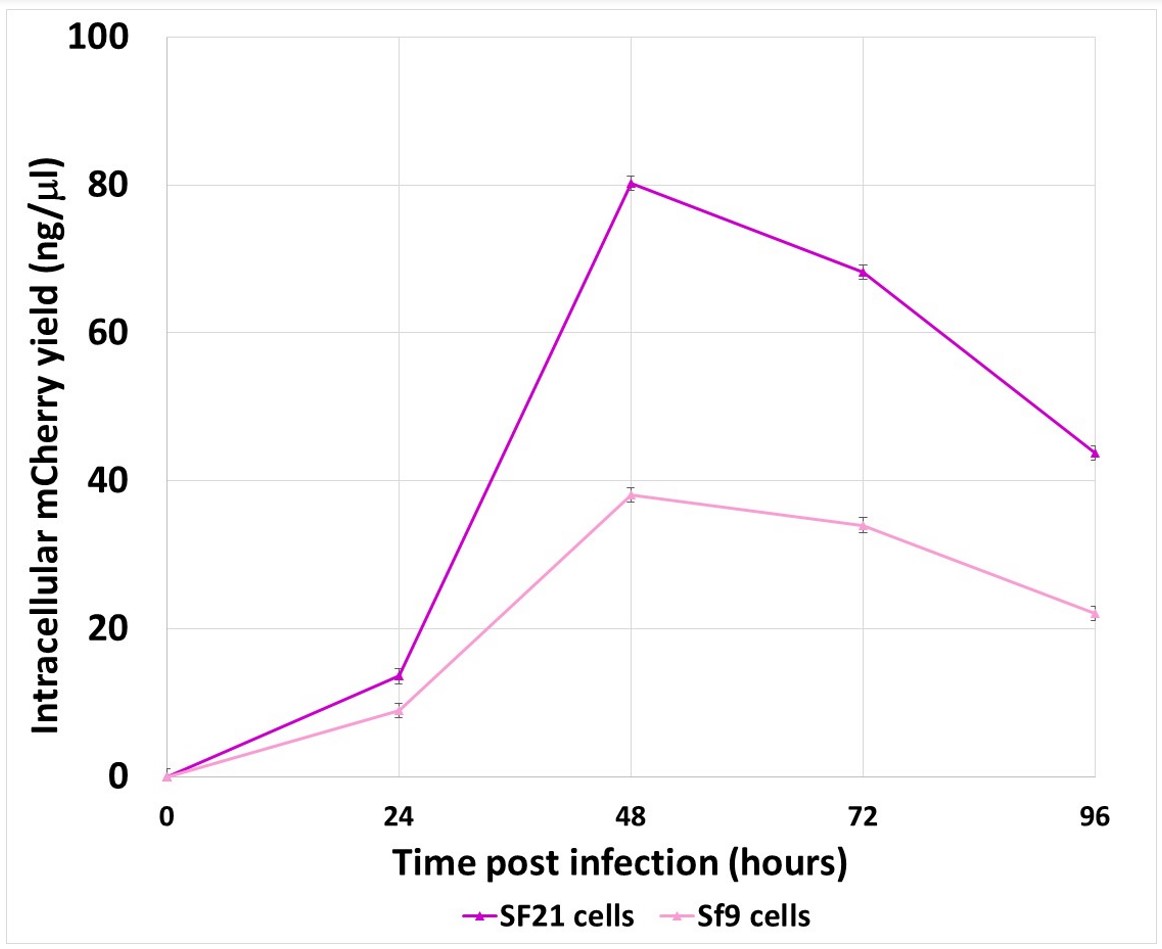
Figure 3: Graph comparing mCherry expression yields between Sf21 and Sf9 cells over 96 hours.
Conclusion: Sf21 cells expressed protein better than Sf9
There is significantly greater intracellular mCherry protein expression in Sf21 cells compared to Sf9 cells validating why Sf21 cells are our insect cell line of choice for intracellular protein expression. We routinely harvest expression cultures 48 hours post infection with recombinant virus and this study confirms this time-point gives optimum protein expression.
Studies with secreted mCherry recombinant virus are now taking place to further investigate optimum cell lines and conditions for secreted proteins in the flashBACTM baculovirus insect cell system. Furthermore, quantification of secreted or intracellular mCherry expression levels are proving to be valuable controls in our mammalian expression systems, highlighting what a powerful tool mCherry protein is in cell biology protein expression studies.


