Chinese Hamster Ovary Cells – A Biologics Powerhouse (past, present & future?)
The first FDA approved recombinant protein therapeutic developed in 1982 was insulin, developed by Genentech/Eli Lilly under the trade name Humulin. E. coli, transformed with recombinant human DNA, was used to express insulin, which was isolated, refolded, and purified. Since the approval in 1986 of the first mammalian-expressed recombinant therapeutic—human tissue plasminogen activator produced in Chinese hamster ovary (CHO) cells—CHO cell lines have become the “go-to” cell line for therapeutic protein production over the last 30 years. The capacity of CHO cells to grow both in suspension culture and at very high cell densities, combined with their adaptability to different growth conditions, has led them to become a mammalian “powerhouse” for producing recombinant proteins at manufacturing scale. However, as biologic drugs increase in complexity, the cellular systems used in their production must evolve to address the various biomanufacturing needs of the future.
The Chinese hamster (Cricetulus griseus) is a rodent that originated in the deserts of northern China and Mongolia (Figure 1).

So why a Chinese hamster?
Chinese hamsters have been used in scientific research labs since the early 1900s where they were used instead of mice for typing pneumococci and in 1948 the Chinese hamster was first used in the US for research into breeding. In 1957, Theodore T. Puck obtained a female Chinese hamster and used this seminal hamster to derive the original Chinese Hamster Ovary (CHO) cell line from epithelial cells of the ovary. The first subclone, CHO-K1, was isolated and distributed by the late 1960s and a new lineage, CHO-S (which was generated simultaneously with CHO-K1) was isolated at Gibco (now ThermoFisher Scientific) during the 1980s. Since that time, many subtypes of these two CHO cell lines have been generated:
- CHO-DXB11 – lacking one allele for dihydrofolate reductase (DHFR), was generated in 1980. CHO-DXB11 allows selection of transfected clones using methotrexate (MTX), which is an inhibitor of DHFR.
- CHO-DG-44 – A CHO line deficient in both alleles of DHFR was isolated in 1983.
Today the commercial CHO expression systems that you can buy are often suspension adapted clones of the original host cell lines and many of these suspension adapted CHO-K1 and DG44 lines have been generated. Examples include CHO-K1 SV (developed by Lonza) for use with the glutamine synthase (GS) expression system, CAT-S (developed by AstraZeneca) and ApolloX CHO-DG44 (developed by Fuji).
More recently, advancements in gene editing technology have been used to generate suspension adapted CHO-K1 cells with single gene deletions. For example, Horizon Discovery working with Merck produced a GS-CHO-K1 knockout, creating CHO-K1 DHFR- and CHO-K1 GS-. A summary of all these CHO lineages is depicted in Figure 2.
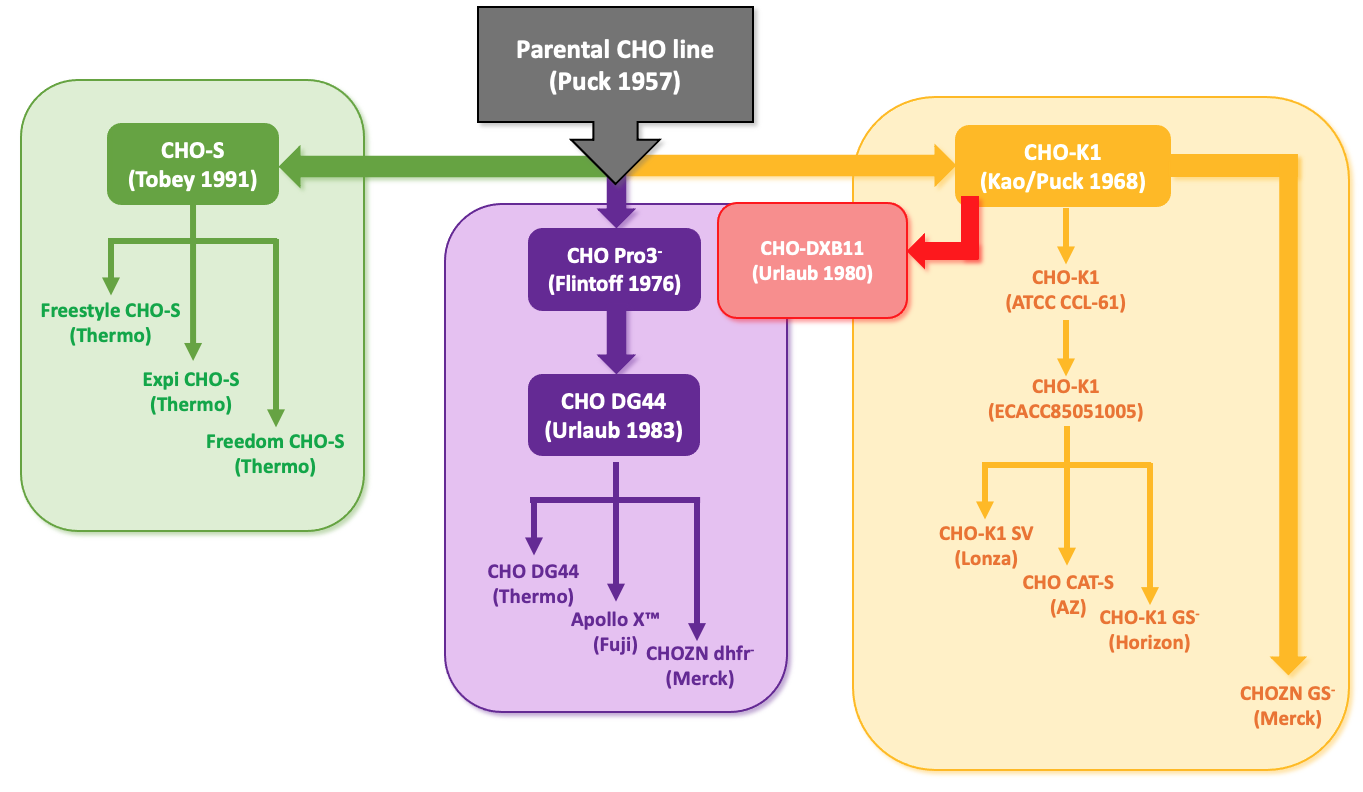
Figure 2: CHO cell lineages
CHO cells are the most common cell line used today for the mass production of therapeutic proteins at industrial scale. Examples of recombinant proteins produced in CHO cells, include hormones, monoclonal antibodies (mAbs), Fc-fusion proteins, bispecific antibodies, Fabs, scfv’s to name but a few.
Why use CHO cells for biotherapeutics?
Several important features of CHO cells make them an attractive choice for biomanufacturing. Firstly, they can grow in suspension culture and in chemically defined (serum-free) media, facilitating the development of precise, controlled, and reproducible manufacturing processes. CHO cells also benefit from a short doubling time (16–22 hours) that rapidly provides the critical cell mass needed to produce recombinant therapeutics at scale. However, the main advantage of CHO cells is in their safety profile, which has been established over several decades and helps ensure compliance with regulatory safety and quality requirements. A further benefit of CHO cells is their ability to replicate post-translational modifications (PTMs), which far surpasses those of commonly used microbial, insect or plant-based expression platforms. Unlike non-mammalian systems, CHO cells can produce almost all human-like PTMs, significantly reducing the potential immunogenic response when the therapeutic agent is administered.
What’s the future for CHO cells?
It will be a long time before CHO cells are replaced as the biomanufacturing system of choice for most of the novel biotherapeutics being developed today. However, it worth noting here that alternative non-mammalian platforms such as fungal, algal and plant systems may be able to offer some of the low-cost solutions that CHO currently cannot.
CHO cells have been the powerhouse of mammalian expression systems since the mid to late 1980s. Back then, it took roughly 12-18 months to make a cell line where the cells grew in serum-containing media with yields roughly around 100mg/L, if you were lucky. Fast forward almost 40 years and we can now generate high cell density and high-protein expressing clonal cell lines in roughly 3-4 months that grow in chemically defined serum free media with protein yields approaching anywhere from 2-10 g/L. During this time the CHO cell genome, transcriptome, proteome and metabolome have been extensively studied and analysed all over the globe and these ‘omics’ datasets have driven the next generation of clonal CHO cell line development.
Therefore, the relevance of CHO cells in the production of future biologic therapeutics will be just as important today and over the next 20 to 30 years just as it has been over the last 40 or so years. The constant emergence of new omics datasets in line with a much deeper understanding of the CHO cell genome will drive this new innovation in CHO cell line development to produce “super expressers” of a whole range of protein modalities in an even quicker and more time efficient manner.
Why we’ve chosen to offer CHO cell recombinant protein expression
At Peak Proteins, we are now offering our clients CHO cell expression as an additional or alternative mammalian expression platform. Our CHO cell expression system uses the CHO-3E7 cell line which utilises the same pTT5 expression vector as our existing HEK293-6E cell line. Both systems, developed in Yves Durocher’s laboratory, are licensed from the National Research Council in Canada. Both cell lines are transient mammalian suspension systems that rely on an EBNA gene expressing EBNA-1 protein in the cell which binds to an oriP sequence in the plasmid to boost expression by nuclear retention and increased episomal replication of the plasmid. By using the same pTT5 plasmid DNA, we can initially screen protein expression in both HEK and CHO suspension systems before deciding on the best expression system to take forward in the project.
At Peak Proteins, we are routinely using E.coli, insect, HEK293-6E and now CHO-E7 mammalian systems, from small ml scale to 25L scale, as part of our Protein Expression Service, normally alongside our protein purification services but occasionally as a standalone expression service. In addition, many of the protein we produced are used in our Protein Crystallography and Structure Determination projects for clients and analysed using our in-house protein mass Spectrometry Service.
If you are interested in any of our services and how they could be used in your research activities, then please contact us directly at info@peakproteins.com for more details.

