Introduction to Detergents for Membrane Protein Solubilisation
By Jade Bowen
At Peak Proteins, we are often asked to purify a variety of complex and challenging proteins. One of the most difficult targets that we work with are membrane proteins, requiring additional steps and the use of detergents during their purification. This blog post will talk about why detergents are used to purify membrane proteins, some of the different types of commonly used detergents, and how we use them.
Why We Care About Membrane Proteins
Membrane proteins, as the name suggests, are located in or associated with cell membranes (Figure 1) and carry out important functions such as signal transduction and transport within and between cells. These proteins allow you to see, feel, think (by facilitating neural firing), and a whole lot more – some of which we don’t even understand yet. As such, membrane proteins are the most common targets for pharmacological intervention, making up around two thirds of drug targets (1). Therefore, studying membrane proteins is essential for developing new drugs, and to do so, membrane proteins must be extracted from cellular membranes and isolated.
Extracting peripheral membrane proteins (Figure 1c) is relatively simple since they are only loosely associated with the membrane and are mostly soluble in aqueous conditions. Integral membrane proteins (Figure 1a &b), however, must be embedded in a membrane to remain folded, in which case the membrane can be emulated by detergents.
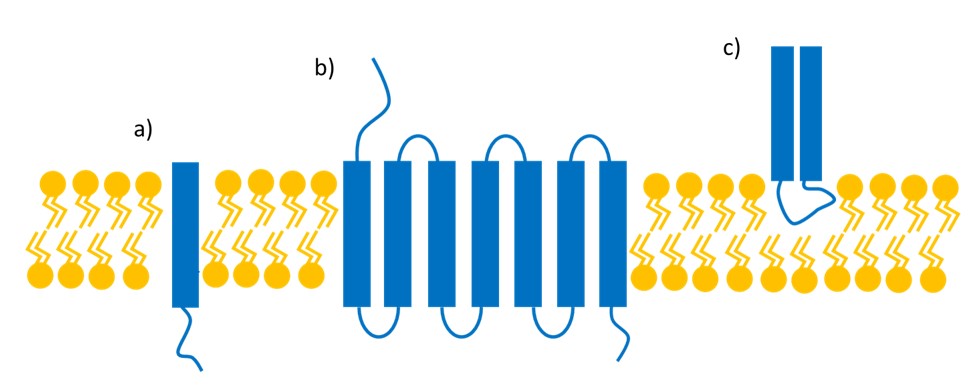
Figure 1: Types of membrane proteins. These can be integral to the membrane and pass the membrane once (a), or multiple times (b), or be peripheral to the membrane (c).
What are Detergents?
Detergents serve to replace the phospholipid bilayer of cell membranes by extracting and maintaining membrane proteins in a folded state in aqueous conditions. They are amphipathic molecules with hydrophilic head groups that interact with water, and hydrophobic alkyl tails that interact with membrane lipids (Figure 2). To successfully extract and solubilise proteins from membranes, detergents must be able to form micelles – spheroid detergent structures with an internal hydrophobic core and an outer shell of hydrophilic head groups (Figure 2c). For micelle formation, the concentration of detergents must be above the critical micelle concentration (CMC) (2), the concentration at which detergent monomers spontaneously assemble into complete micelles. Not all detergents are created equal, however, with some more suited to specific protein classes or experiments than others. The detergents we use largely fall into the following categories: maltosides, glucosides, neopentyl glycol and steroidal detergents, some of which we will explore below.
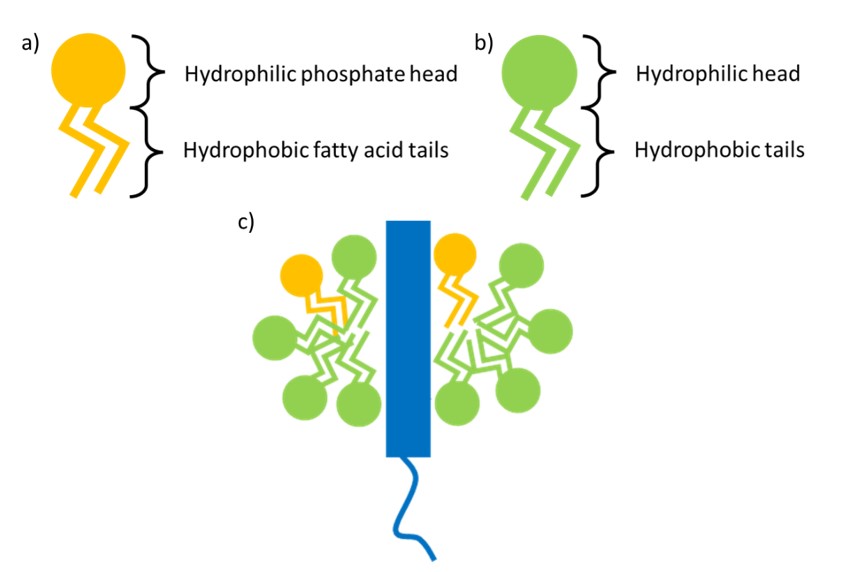
Figure 2: a) Phospholipid structure. b) Detergent structure, note: the number and length of alkyl tails varies between detergents. c) Structure of a membrane solubilised in a detergent micelle
OG
Octyl-beta-glycoside (OG, Figure 3) is a classic glucoside detergent used heavily in the 80’s for membrane protein biochemistry (3). This detergent is still commonly used today but has lost its popularity due to its high CMC of ~20 mM (4), requiring high amounts to maintain the solubility of membrane proteins during purification, and the fact that it is relatively harsh toward more sensitive membrane proteins such as GPCRs (G-protein coupled receptors).
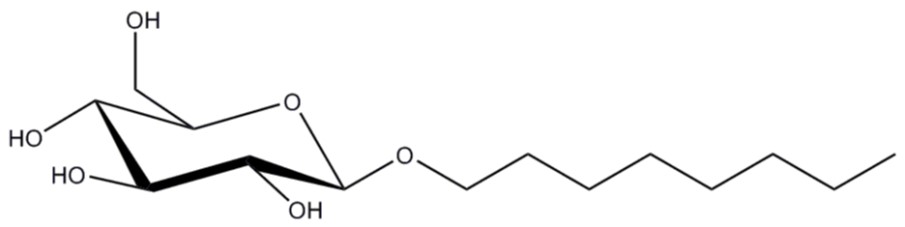
Figure 3: Structure of octyl-beta-glycoside (OG) with a short hydrophobic tail and one hydrophilic sugar head group.
DDM
Maltosides are one of the most widely-used classes of detergents for solubilising most types of membrane proteins (5). Dodecyl maltoside (DDM), is one of our go-to detergents (Figure 4). This detergent has a lower CMC of 0.15 mM (6), meaning that less is required in buffers after solubilisation, and it is gentler on sensitive membrane proteins than OG, hence its popularity. However, some membrane proteins are still unstable in DDM, therefore require other additives such as cholesterol hemisuccinate (CHS), a cholesterol mimic that is often used with DDM to increase stability of fragile membrane proteins.
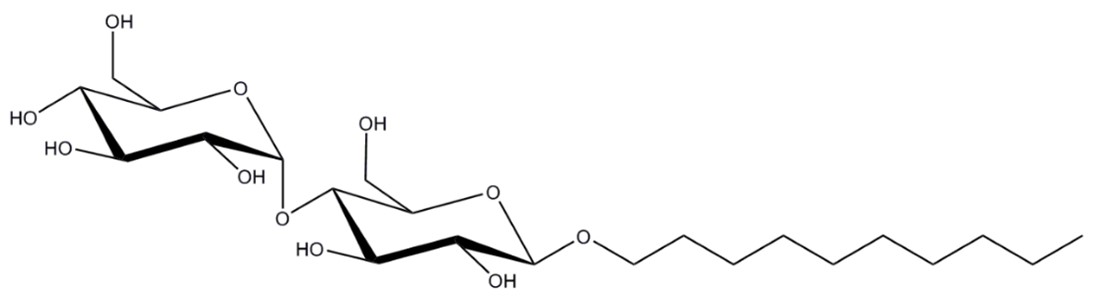
Figure 4: Structure of DDM (dodecyl maltoside), with a hydrophilic maltose head group and hydrophobic alkyl tail.
LMNG
One of the next most commonly used detergents at Peak Proteins is lauryl maltose neopentyl glycol (LMNG). This detergent has two hydrophobic tails (Figure 5) which helps to stabilise more delicate membrane proteins by packing densely around the protein thereby preventing them from unfolding in the micelle (7). The CMC is incredibly low, and the off-rate for detergent leaving LMNG micelles is slow, meaning that it can be used in vanishingly small amounts in purification buffers, which can be an advantage for downstream biophysical assays and structural studies.
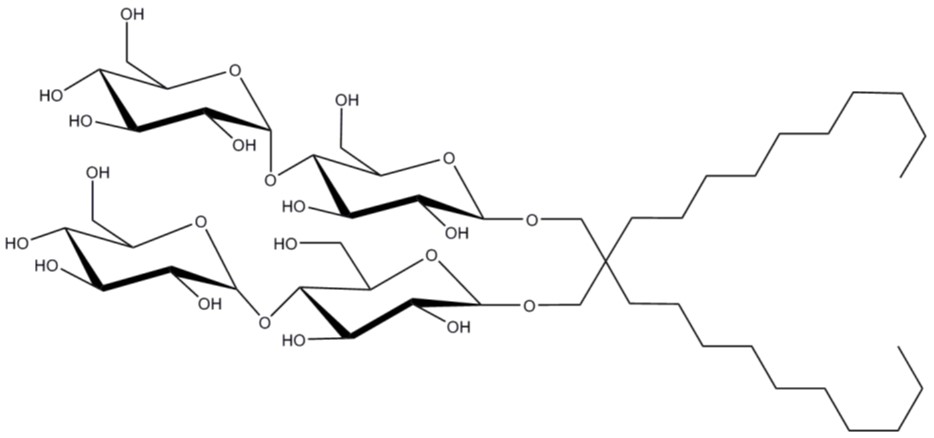
Figure 5: Structure of LMNG (Lauryl maltose neopentyl glycol) with two hydrophilic sugar heads and two hydrophobic tails.
Digitonin and GDN
Sometimes we might find a protein solubilises well in a certain detergent, but that the detergent isn’t suitable for our client’s needs. In this case, we need to find a detergent that works well for both the protein and the client. For example, digitonin (Figure 6), a highly toxic steroidal detergent, and its synthetic non-toxic counterpart, GDN (glyco-diosgenin), are often used for structural studies like cryo-EM because they form defined micelles, increasing the likelihood of a higher resolution structure being obtained (8).
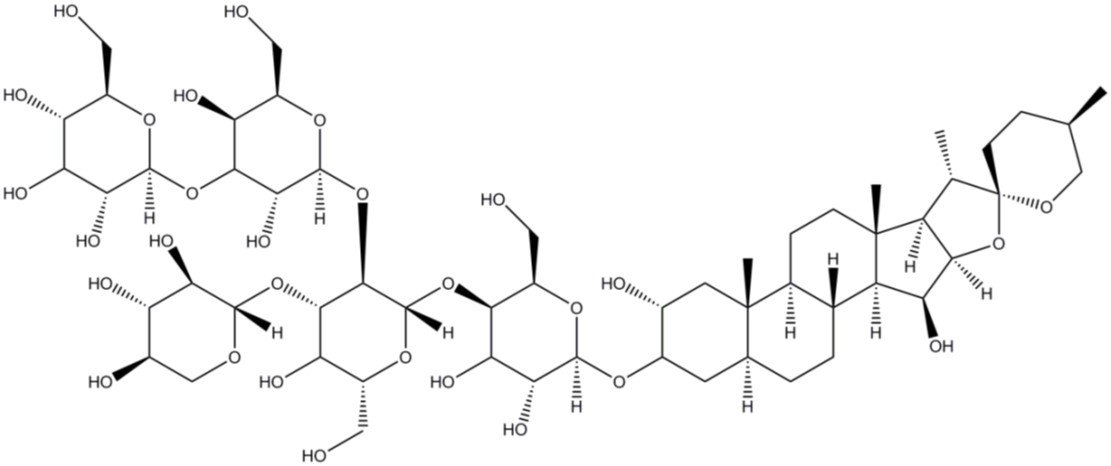
Figure 6: The structure of digitonin – a large, complex steroidal detergent.
Novel Detergents
One promising new class of detergents are asymmetrical maltose neopentyl glycols (A-MNGs) – these have a similar overall structure to LMNG (Figure 5), but the hydrophobic tails are different lengths leading to their asymmetry (9,10). The difference in tail length allows these detergents to pack tightly, creating a smaller micelle than LMNG, and have been shown to stabilise GPCRs more effectively. This class of detergents are not currently on the market, but as soon as they are we will be excited to test their efficacy for solubilisation, stabilisation, and suitability for downstream analysis.
Conclusion
This is not an exhaustive list of detergents used here at peak proteins, and there is a large selection of novel detergents with variable structures that may perform well for a particular protein. Non-detergent based approaches are also available and offer their own unique benefits (11), such as SMALPs, discussed in a previous blog post. Proteins are delicate and behave in their own unique way, especially membrane proteins. Therefore, the best approach to successfully purify them often needs to be arrived at empirically. One method of selecting the best detergent for the membrane protein in question, and ensuring the protein is in their most favourable conditions for our client’s needs is detergent screening. If you would like to learn more about how we can tackle your challenging membrane protein targets, please get in contact with our team of specialists. info@peakproteins.com
References
1. Errasti-Murugarren E, Bartoccioni P, Palacín M. Membrane Protein Stabilization Strategies for Structural and Functional Studies. Membranes (Basel). 2021;11(2):155. Published 2021 Feb 22. doi:10.3390/membranes11020155
2. Stetsenko A, Guskov A. An Overview of the Top Ten Detergents Used for Membrane Protein Crystallization. Crystals. 2017; 7(7):197. doi:10.3390/cryst7070197
3. Hjelmeland LM, Chrambach A. [16] Solubilization of functional membrane proteins. Methods in Enzymology. 1984;104(1): 305-318 Published January 1, 1984. doi:10.1016/s0076-6879(84)04097-0
4. Octyl-beta-Glucoside Detergent. www.thermofisher.com. Accessed February 29, 2024. https://www.thermofisher.com/order/catalog/product/28310?SID=srch-srp-28310
5. Choy BC, Cater RJ, Mancia F, Pryor EE Jr. A 10-year meta-analysis of membrane protein structural biology: Detergents, membrane mimetics, and structure determination techniques. Biochim Biophys Acta Biomembr. 2021;1863(3):183533. doi:10.1016/j.bbamem.2020.183533
6. n-Dodecyl β-D-maltoside. www.sigmaaldrich.com. Accessed February 29, 2024. https://www.sigmaaldrich.com/GB/en/product/sigma/d4641
7. Lee S, Ghosh S, Jana S, Robertson N, Tate CG, Vaidehi N. How Do Branched Detergents Stabilize GPCRs in Micelles?. Biochemistry. 2020;59(23):2125-2134. doi:10.1021/acs.biochem.0c00183
8. Li S. Detergents and alternatives in cryo-EM studies of membrane proteins. Acta Biochim Biophys Sin (Shanghai). 2022;54(8):1049-1056. doi:10.3724/abbs.2022088
9. Ratkeviciute G, Cooper BF, Knowles TJ. Methods for the solubilisation of membrane proteins: the micelle-aneous world of membrane protein solubilisation. Biochem Soc Trans. 2021;49(4):1763-1777. doi:10.1042/BST20210181
10. Lee HJ, Lee HS, Youn T, Byrne B, Chae PS. Impact of novel detergents on membrane protein studies. Chem. 2022;8(4): 980-1013. doi:10.1016/j.chempr.2022.02.007
11. Young JW. Recent advances in membrane mimetics for membrane protein research. Biochem Soc Trans. 2023;51(3):1405-1416. doi:10.1042/BST20230164

