Purifying 5-HT2AR GPCR for cryo-EM Structural Studies
Ben’s PIPs Placement Journey with Peak Proteins
In this article PhD Student Ben Nash discusses his 3-month PIPs placement work at Peak Proteins, which involved the expression and purification optimisation of the G-protein coupled receptor (GPCR) 5-HT2AR. Ultimately, Ben’s work has set us on a path towards cryo-EM structural studies of this target.
Here is Ben’s story:
Hello! I am Ben, a PhD student at the University of East Anglia (Norwich) where my PhD research focuses on understanding the structure and properties of bacterial heme proteins. These proteins allow them to respire without oxygen and interest us for their potential use in biotechnologies like biomining and bioremediation. The Norwich research park PhD program includes a three-month PIPS (Professional Internships for PhD Students) placement period which students like myself must undertake during their second or third years, with the topic of the placement work being left entirely to the student’s choice.
I first heard about Peak Proteins through a colleague who recommended the company to me as a potential host with whom I would be a good fit for my PIPS. After a little investigation I discovered the skills I had been developing throughout my PhD, purifying proteins and determining their structures, were highly valued at Peak where they are two of the most important services offered to their clients.
I got in touch with Dr Steven Harborne about whether they were interested in discussing me undertaking a placement there and soon enough I was packing my bags for Macclesfield. I was both excited and quite apprehensive about moving to a new laboratory, to work in a new team and on a totally new project, and also a little sad to be dropping my existing research project for a while.
I was quickly welcomed into the membrane protein team at peak where we set about aiming to purify the 5-HT2AR GPCR with the eventual goal of determining its structure by cryogenic electron microscopy (cryo-EM) as a benchmark structure. 5-HT2AR made for a great choice as there was good precedent in the literature for determination of its structure both by X-ray crystallography and cryo-EM, and it’s a potentially promising target to treat neuropsychiatric disorders [1–3].
We began by testing the effectiveness of different expression vectors by two techniques that were new additions to my skillset, FSEC (using a fluorescently labelled antibody fragment) and western blotting. Once we identified a promising candidate, we scaled up to a 1 L grow where I was able to get hands on with the growth and transfection of insect cells. Using insect cell expression systems is common for GPCRs but was completely outside of my experiences in cell culture which were limited to bacterial systems. Through affinity chromatography and size-exclusion chromatography we were able to isolate 5-HT2AR in complex with a homotrimeric G-protein complex which was visualised by SDS-PAGE and its identity confirmed by peptide mapping mass spectrometry using Peak Proteins’ in house LCMS facility.
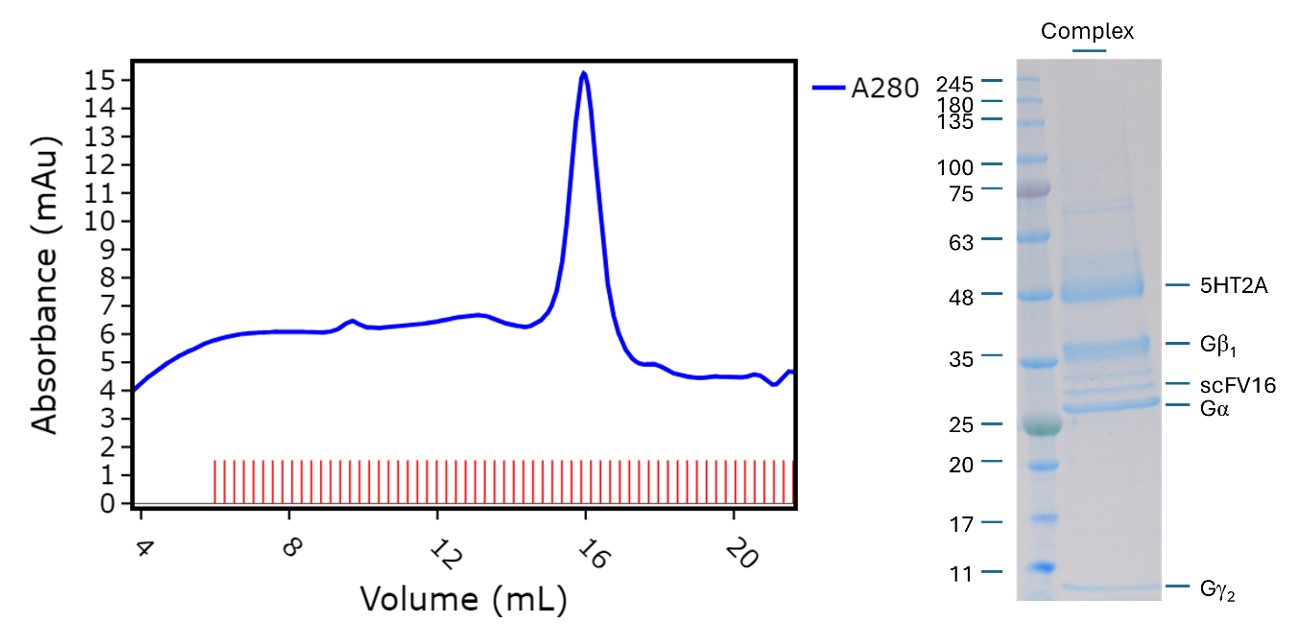
Figure 1. SEC Trace for 5HT2A GPCR:G-protein complex and reducing SDS-PAGE analysis of isolated GPCR:G-protein complex
With pure receptor:G-protein complex in hand, we used FSEC to test our sample’s ability to bind the antibody fragment ScFv16 which provides necessary stabilisation and mass for cryo-EM and happily found it capable of doing so. We were also able to confirm its stability upon transfer into the detergents GDN and LMNG which are often popular choices when preparing cryo-EM grids of membrane proteins.
Up to this point, we had been expressing our 5-HT2AR in Spodoptera frugiperda cells, but with there being literature precedent also for expression in High 5 (Trichoplusia ni) insect cells we wanted to test the effect of swapping insect cell lines [2,4,5]. We repeated our purification procedure and were able to produce a product with comparable yield and purity from this cell line too.
Hopefully, these results will provide firm groundwork for the continued goal of determining a cryo-EM 5-HT2AR structure. Importantly for me, my time with Peak Proteins also provided an opportunity to gain completely new skills such as western blotting, and perfect existing ones like use of AKTA purification systems. I made lots of new friends, and I was also exposed to many ways in which industry science differs from academic science, making me feel more informed as to where I go next in my career after my PhD. Having now concluded my PIPS placement, I’m a much more confident scientist than I was starting it and for that reason I am very grateful that the placement scheme is offered by my PhD program and that through it, I found my way to Peak Proteins.
References
1. Kaplan, A. L. et al. Bespoke library docking for 5-HT2A receptor agonists with antidepressant activity. Nature 2022 610:7932 610, 582–591 (2022).
2. Kim, K. et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 182, 1574-1588.e19 (2020).
3. Kimura, K. T. et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nature Structural & Molecular Biology 2019 26:2 26, 121–128 (2019).
4. Wang, X. et al. Molecular insights into differentiated ligand recognition of the human parathyroid hormone receptor 2. Proc Natl Acad Sci U S A 118, e2101279118 (2021).
5. Cao, C. et al. Structure, function and pharmacology of human itch GPCRs. Nature 2021 600:7887 600, 170–175 (2021).


