The Magnetic Attraction of Protein NMR – and why you might be drawn to it!
Written by Dr Roberto Maya
Here at Peak Proteins, we have a long history of expressing and purifying labelled proteins for our clients to support their NMR studies. With Dr. Roberto Maya, an NMR specialist, joining our team, coupled with access to NMR magnets and facilities, we are really excited that we are now in a position to offer NMR data collection and interpretation to our clients.
The facility collaborations we have are with the Astbury Centre at Leeds University, and also the Biomolecular NMR facility at Birmingham University; the latter through an existing link via Sygnature Discovery, led by our colleague Juliet Morgan.
The NMR services we are currently offering are:
- Production of uniform and specifically labelled proteins
- Analysis of stable and transient protein : ligand interactions
- Protein quality control and stability measurements
If it is of interest, we can also support other NMR applications such as structure determination, following kinetic process in real time and backbone and methyl assignments.
In this first of a (sporadic) series of blogs, Roberto introduces NMR and takes a look at how it can be used to explore the folded state of a protein and to investigate small molecule protein interactions.
First things first – what actually is NMR?
Nuclear Magnetic Resonance (NMR) is a versatile biophysics technique that allows us to explore the properties of molecules in solution. The molecules themselves can range from small molecules, proteins, carbohydrates through to nucleic acids. There is also no size restriction or limit for NMR studies and molecules size can span from a few Daltons up to mega Daltons.
The principle behind NMR is that nuclei have spin and are all electrically charged. If an external magnetic field is applied, energy transfer is possible between the base energy level to a higher energy level. The energy transfer takes place at a wavelength that corresponds to radio frequencies and when the spin returns to its base energy level, energy is emitted at the same frequency. These signals are then measured and processed to yield an NMR spectrum for each nucleus. NMR was first detected experimentally in 1945, by Felix Bloch at Stanford University and Edward Purcell at Harvard University and both were awarded the 1952 Nobel Prize in Physics for their research in this field.
In the 1960s, the application of superconducting magnets and computers to NMR equipment led to a huge improvement in sensitivity, allowing the study of more complex proteins, such as membrane proteins, metabolically complex samples, or even biological tissues. As a result, NMR spectroscopy has become an incredibly powerful technique for the study of protein molecular dynamics, interactions and structural resolution.
There are two basic requirements to explore molecules by NMR; firstly having an NMR spectrometer and secondly, for some applications, having labelled proteins.

NMR spectrometer at the Astbury Centre, Leeds University
The NMR instrument is basically an electronic device that generates an immense magnet field (250,000 times higher than the earth’s field) that allows the detection of the micro-fields generated by the nuclear spin of the active atoms within the molecules. It is important to highlight that ¹⁵N, ¹³C and ¹H are some the atoms that are magnetically active in NMR. However, the natural abundance of ¹⁵N and ¹³C in biological molecules is extremely low: 0.3% (¹⁵N), 1.1% (¹³C), whereas ¹H is high at 99.9% . With that in mind, we often aim to boost the levels of ¹⁵N and ¹³C in target proteins by incorporating those isotopes during recombinant protein production. This is normally achieved in bacterial expression systems by supplementing a minimal cell culture media with the specific stable-isotope labelled amino acids, glucose, ammonia and vitamins needed for cell growth.
Quality control and stability of proteins
For unlabelled proteins, we can take advantage of the natural abundance of ¹H, which is magnetically active for NMR and widely distributed in proteins. With 1D-NMR spectrum, it is possible to acquire all the protons from the target protein: HN, CH, CH2 and CH3. In particular the HN and the CH3 are excellent sensors to describe the overall packing of the protein (1). In a well folded protein, these resonances are well dispersed in the spectra. For partially or completely unfolded proteins, the HN resonances collapse to a marginal region between 7.5 and 8.5ppm in the 1H frequency (Figure 1A below).
If the protein is ¹⁵N and/or ¹³C labelled, the characterisation is rationalised to be residue specific. The dimensions are increased from 1D to 2D, where H/N, H/C or N/C atoms can be correlated. H/N is the most widely used for its simplicity in terms of resonances overlapping. For example, the H/N-NMR spectrum of a protein will show only one resonance per amide group. No assignment is required for the analysis of the data; however, it is indispensable for further characterisation (Figure 1B).
In addition, the 2D NMR spectrum serves as a template to evaluate binding, dynamics, backbone assignment, and structure determination in proteins.
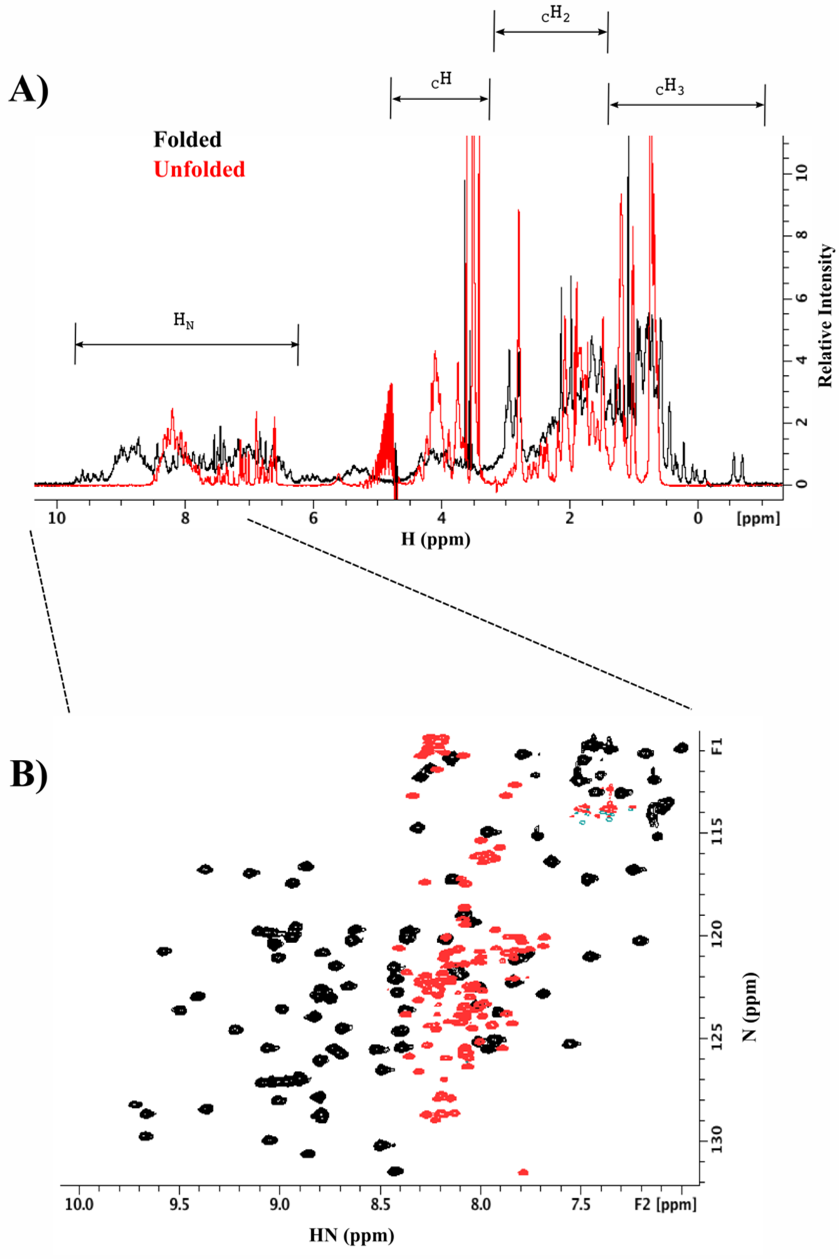
Figure 1. 1D and 2D-NMR spectra of folded (black) and unfolded proteins (red). A) According to the nature of the protons, they experience a specific frequency in the H dimension; see highlighted regions on top of the figure. B) Increasing the dimensionality of the amide region (H/N correlation spectrum).
Evaluating interactions among molecules
NMR is the only biophysics technique that allows the evaluation of interactions among molecules in solution with atomistic resolution. In the last two decades, NMR has been widely used in drug discovery for fragment screening and hit validation, due to its versatility and ability to detect a wide range of affinities from mM to nM. There are two ways to evaluate the interactions between molecules depending on their nature:
1. Ligand observed
In this strategy, the small molecule serves as sensor to detect the binding with its target, normally a protein. Labelling of the protein is therefore not necessary for this approach and there is no upper limit to the size of the protein. A further advantage is that a mixture of compounds can be evaluated at the same time.
Having labelled the small molecule, there are three, well described methodologies used to detect binding to the small molecule.
1. Saturation Transfer Difference (STD): here, the most distant HN and/or CH₃ resonances of the protein are excited and then the active magnetisation is transferred through the rest of the protein and also to any small molecules that are stably interacting with it. As result, only the positive binders will show a representative resonance in the 1D spectrum (Section A Fig. 2) (2).
2. Water-Logsy: in this strategy, the water molecules of the solvent are excited and then the water transfers its magnetization to the small molecules that are in contact with the protein by a phenomenon known as Nuclear Overhauser Effect (NOE). It is important to mention that only the water molecules that are trapped in the binding interface are capable of transferring their magnetization to the small molecules, due to a reduction in their free diffusion from being trapped in the binding cavity (2). Only positive binders will induce a peak inversion of the resonances from the atoms that are involved in the interaction (Section B Fig.2).
3. ¹⁹F-FAXS: This method takes the advantage of the 19Fluoride atom which is magnetically active in NMR and will depend on the number of ¹⁹F atoms presents in the small molecule. In binding assays, the ¹⁹F resonances of positive binders will experience perturbations in their local environment that will result in shifts with respect to the reference control (Section C Fig. 2) (3).
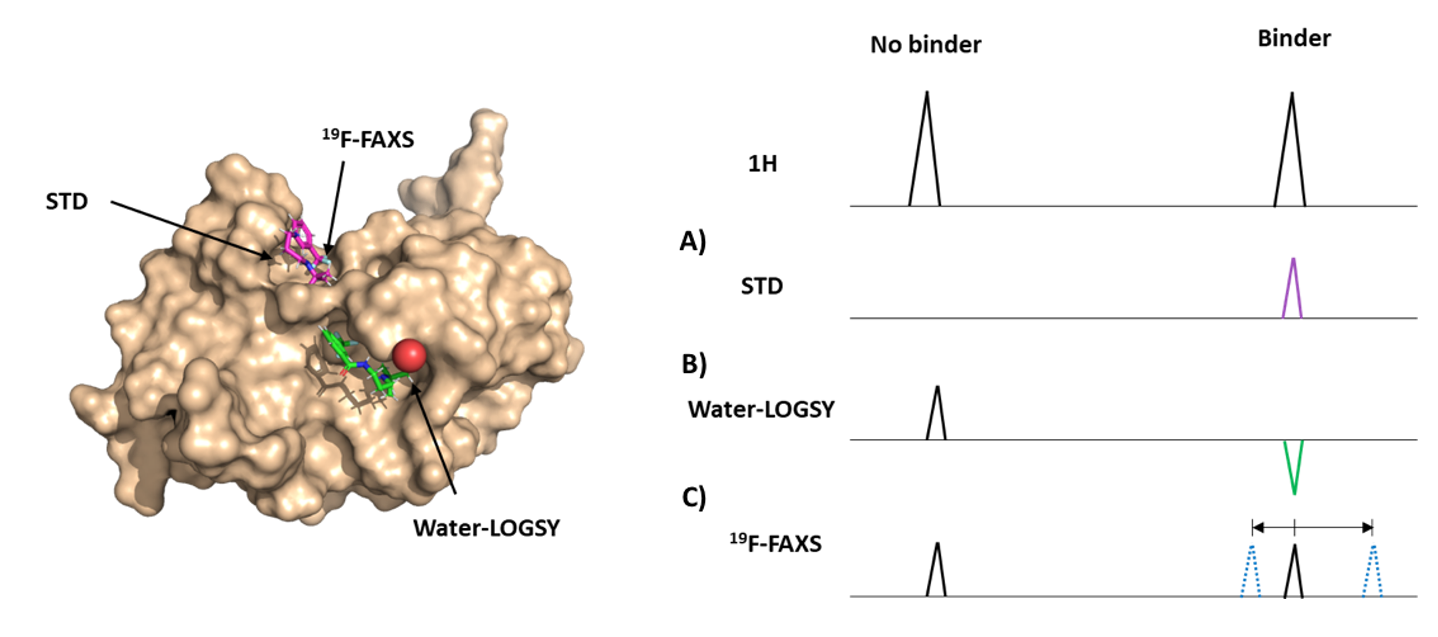
Figure 2. Methodologies to explore binding process between small molecules and proteins. On the left panel is the 3D representation of the molecules involve in the interaction. On the right panel is the graphical representation of the expected resonances for no binders and binders, for each methodology.
2. Protein observed
To apply this methodology, the target protein needs to be uniformly or specific enriched with ¹⁵N or ¹³C isotopes. For the first screening approach, to detect binding patches, the assignment of the target protein is not required. The nature of the ligands can be widespread from small molecules, peptides, nucleic acids, carbohydrates, proteins and even lipids.
2D HN-NMR spectrum is the most widely used for detecting stable or transient interactions. Chemical Shift Perturbation (CSP), linewidth (LW) and intensity/volume (I/V) of the HN resonances are measured and calculated to evaluate the binding affinity between molecules (4). Additionally, it is possible to determine the dissociation constant per residue involved in the interaction.
CSP, LW and I/V data are normally used as experimental constraints to determine 3D models of the ternary complex (Fig. 3).
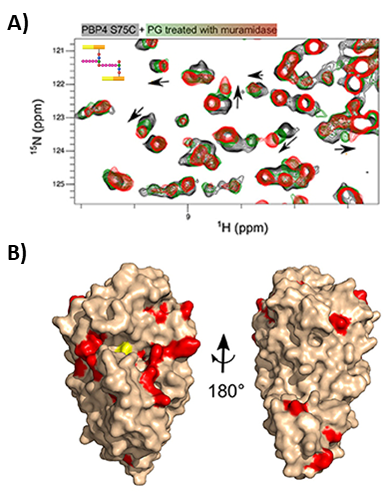
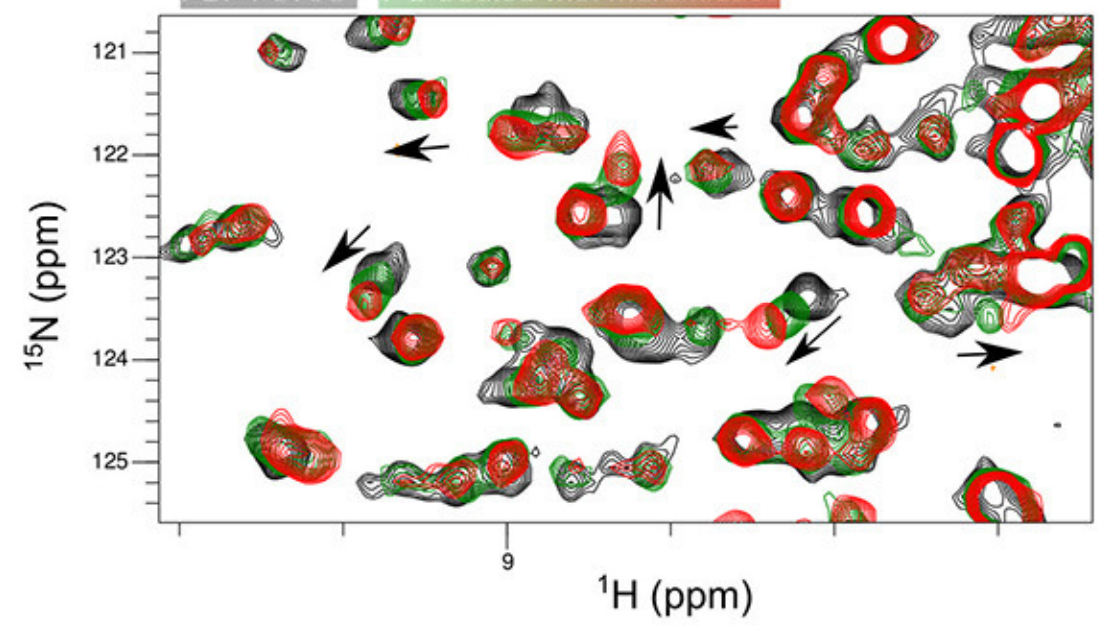
Figure 3. Extracting structural information of protein ligand interactions from NMR. A) Example of ligand titrations followed by H/N-2D NMR spectra. Trend of the residues involved in the interaction are shown by black arrows. B) 3D reconstruction model based on CSP restraints obtained from A). From Maya-Martinez et al. Front. Microbiol. 18(9) (2019)
If you have a project that you think would benefit from a protein NMR approach, then please do get in touch with us and we’d be happy to discuss. info@peakproteins.com
References
1. P. Schanda, V. Forge, B. Brutscher, HET-SOFAST NMR for fast detection of structural compactness and heterogeneity along polypeptide chains. Magn. Reson. Chem. 44 (2006).
2. C. Raingeval, et al., 1D NMR WaterLOGSY as an efficient method for fragment-based lead discovery. J. Enzyme Inhib. Med. Chem. 34, 1218–1225 (2019).
3. R. S. Norton, E. W. W. Leung, I. R. Chandrashekaran, C. A. MacRaild, Applications of 19F-NMR in fragment-based drug discovery. Molecules 21 (2016).
4. M. P. Williamson, Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 73, 1–16 (2013).
5. Maya-Martinez, et al., Recognition of Peptidoglycan Fragments by the Transpeptidase PBP4 From Staphylococcus aureus. Front. Microbiol. 18(9) (2019)