The Role of Structural Biology in the Development of Biotherapeutics
In recent years, structural biology has become more prevalent in biotherapeutic development playing a pivotal role in elucidating the antibody-antigen complex formation and understanding the effect on structure of introducing amino acid substitutions. By employing techniques such as X-ray crystallography, NMR spectroscopy, and cryo-EM, structural biologists can visualize the three-dimensional structures of antibodies and their complexes with antigens to near-atomic resolution.
By examining intermolecular interactions at the complex interface, we can characterize the which residues are key for binding and specificity. This knowledge is key for engineering antibodies to optimise binding affinity and minimise impact of sequence liabilities in the Complementarity-determining regions (CDRs). Detailed antigen-antibody complex structures can allow us to determine whether conformational changes occur within the antigen after complex formation providing mechanistic insights1 . Furthermore, 3-dimensional structures aid in predicting the stability of antibody therapeutics. Structural studies also provide insights into the determinants of antibody stability, areas of hydrophobicity and sequence liabilities. These data can be used to optimize antibody formulations for improved pharmacokinetics and shelf-life2.
Structures determined by X-ray crystallography still make up the majority, <90 %, of the ~3000 available antibody related structures in the PDB of submission (including antibody/fragments, antigen-antibody complexes)2. However, the size, flexibility and glycosylation of antibodies results in them being very difficult to crystallise. Currently all antigen- antibody complex deposits resolved by crystallography utilise an antibody fragments such as Fabs, ScFvs and nanobodies, there are no full-length antibody- antigen complex structures available. This is the primary limitation of X-ray crystallography in biotherapeutic development.
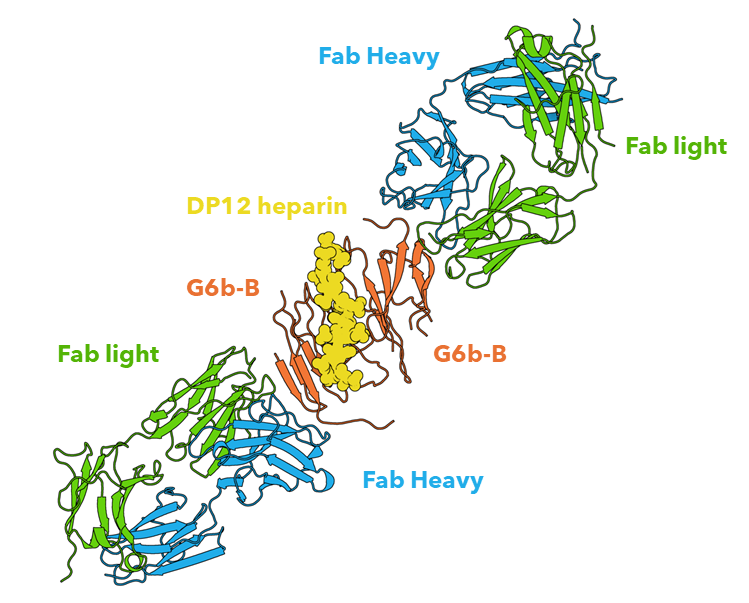
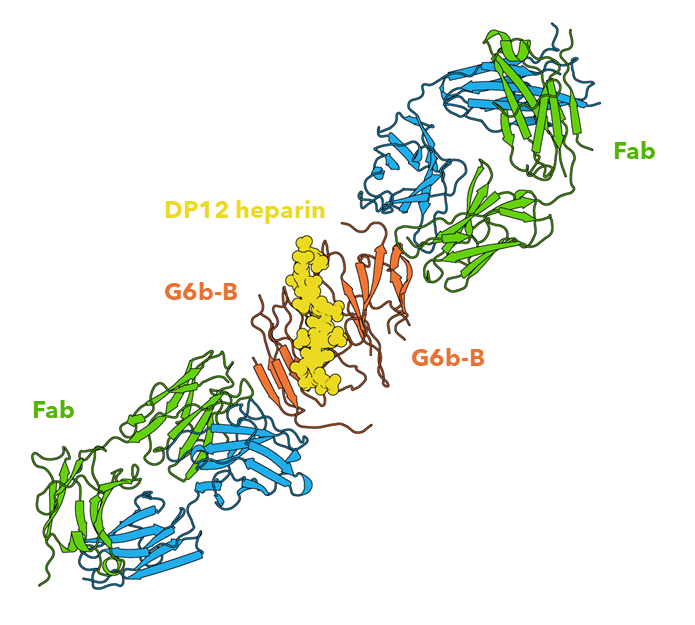
Figure 1: The extracellular domain of G6b-B in complex with Fab fragment and DP12 heparin oligosaccharide. Structural work performed by Peak Proteins scientists3.
The developments of Cryo-EM, allowing for higher resolution structure determination, has led to a significant increase in its use in biologic development as it has several benefits over X-ray crystallography. This is primarily due to its ability to resolve multi sub-unit complexes without requiring crystallisation, an attribute that makes it ideal for resolving antibody-antigen complexes4,5. Further to this, antigens are required to be present of the cell surface of proteins which results in almost all antigens being membrane proteins which contain a hydrophobic transmembrane region which significantly impedes crystal formation. Therefore, Cryo-EM has become the primary technique for structurally resolving membrane proteins, thus increasing the number of structures available of potential biologic targets with some ground being made into resolving bispecific antibody- dual antigen engagement4–7.
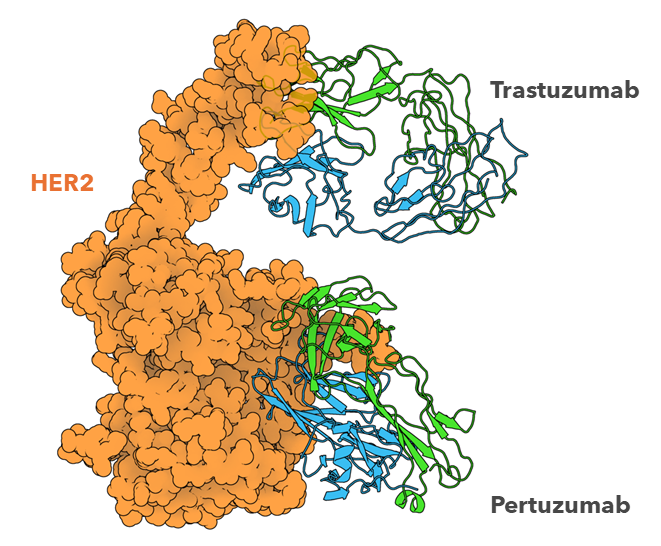
Figure 2: Cryo-EM structure of HER2-trastuzumab-pertuzumab. Trastuzumab and pertuzumab are monoclonal antibodies that bind to distinct subdomains of the extracellular domain of human epidermal growth factor receptor 2 (HER2). Comparison of this ternary complex to the individual binary complexes reveals no cooperative interaction between trastuzumab and pertuzumab, and provides key insights into the design of novel, high-avidity bispecific molecules with potentially greater clinical efficacy5.
NMR has not been utilised to the same extent as X-ray crystallography and Cryo-EM as it is limited by an upper limit on molecular weight (<30 kDa). Yet there are benefits for utilising NMR for smaller biologic molecules such as nanobodies and ScFvs. NMR spectroscopy is dynamic and is undertaken in solutions and therefore crystallisation is not required. NMR has also been used successfully to characterise biotherapeutic formulation development8. Furthermore, NMR has been used to map epitopes successfully and is particularly successful at determining competitive binders. NMR is limited however by its requirement for isotopically labelled protein by either 13C or 15N which are primarily produced in E.coli expression systems9,10.
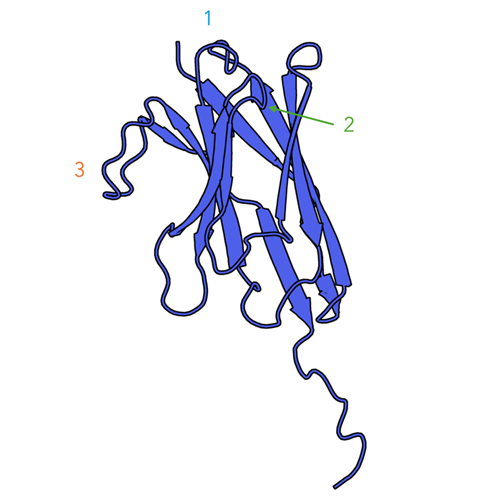
Figure 3: Solution structure of camelid α-aflatoxin B1 nanobody Nb26 resolved by NMR spectroscopy(PDB 6IYN). CDR loops have been mapped Using IMGT and Labelled accordingly11.
In summary, structural biology offers a molecular-level understanding of antibody-antigen interactions, providing valuable insights into the design, optimization, and application of antibody therapeutics. By unravelling the structural intricacies of antibody-antigen complexes, structural biologists contribute to the development of more effective and targeted therapies for a wide range of diseases.