Tricks and Tips to Maximise Protein Expression in Mammalian Cells
In this article our Cells Team share some tips and tricks for improving the yields of recombinant proteins expression in mammalian cells
An earlier blog outlined the benefits of using mammalian cells as an expression host. At Sygnature Discovery we currently offer HEK293-6E and CHO-3E7 (licensed from the National Research Council of Canada) and ExpiCHO-S and Expi293F (Thermo Fisher Scientific) expression systems for transient mammalian protein production at scales up to 25 L.
In the HEK293-6E and CHO-3E7 cell lines the use of the stably integrated Epstein-Barr virus (EBV) nuclear antigen (EBNA-1) along with pTT5 expression plasmids containing the EBV oriP sequence leads to enhanced replication and retention of the plasmid and ultimately higher expression levels over a prolonged period of time. High expression levels of proteins such as antibodies, Fc fusions and cytokines can give the impression expression in mammalian cells is fairly straightforward. Of course, no expression system works perfectly and no matter how well optimised your transient expression system is, some proteins remain difficult to express and can commonly give expression levels as low as <100 µg/L. Attempting to resolve this problem can be costly, time consuming and very frustrating. So, what causes low expression and how can you improve it?
The production of recombinant proteins in mammalian cells can potentially be affected by limiting steps along the protein expression pathway e.g. efficiency of gene transcription, translation and post-translational processing (Hussain et. al., 2014). Therefore, approaches which address these ‘bottlenecks’, such as slowing down the process of transcription or translation so gene expression matches the secretory capacity of the host cell, can be beneficial.
A commonly used method is to reduce the gene copy number for your recombinant protein. The gene of interest is titrated down and carrier DNA added to the mixture to maintain the total amount of DNA being transfected at a constant level. Figure 1 shows how this technique was applied to the expression of GFP-tagged Excitatory Amino Acid Transporter 2 (EAAT2) membrane protein to identify the optimum percentage of EAAT_pTT5 plasmid and carrier DNA for subsequent larger scale transient transfection expression grows. Expression of EAAT2 protein was assessed using fluorescent size-exclusion chromatography (FSEC).
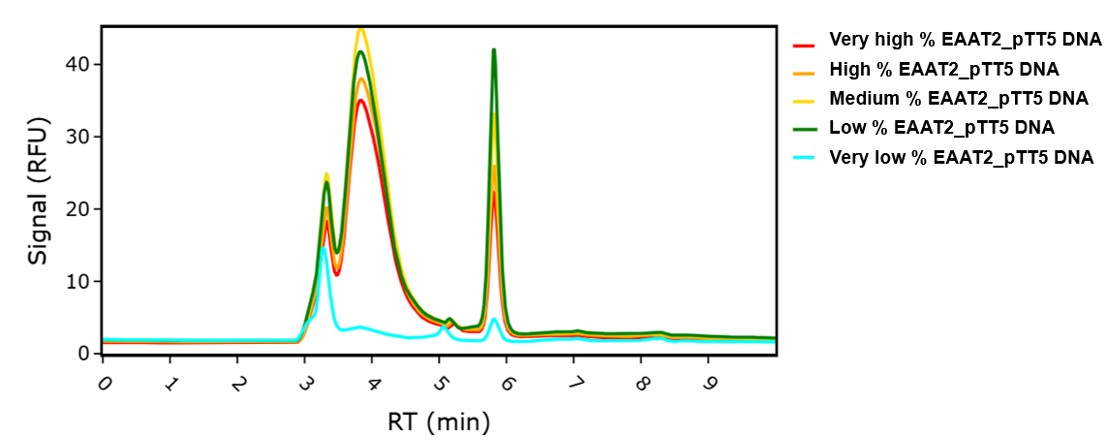
Figure 1: FSEC data showing expression of GFP-tagged EAAT2 membrane protein in HEK293-6E cells for different ratios of coding and carrier DNA.
It is important to note that the choice of carrier DNA may be a significant factor. Estes et. al., 2014, show that when trying to improve the quality of secreted proteins prone to aggregation in HEK293 cells, using an empty version of the transfection vector as carrier DNA gave better results than sheared genomic DNA or a different vector with a similar supercoiled plasmid form but lacking eukaryotic regulatory elements. Their hypothesis is that vector elements in the carrier DNA are essentially acting as a decoy for transcription factors or other regulatory elements and thereby decreasing the rate of transcription for the gene of interest. This not only decreased levels of aggregated protein produced but led to better signal peptide cleavage and more homogeneous N-linked glycosylation profiles.
The use of mildly hypothermic expression conditions has also been widely reported to have a positive effect on recombinant protein production and is a simple method to implement. However, the beneficial effect of low culture temperature on expression appears to be cell-type-specific and time dependent so appropriate optimisation is required. Figure 2 demonstrates the beneficial effect of a temperature-shift 24 h post transfection on the expression of the fluorescent intracellular mCherry protein in ExpiCHO-S cells.
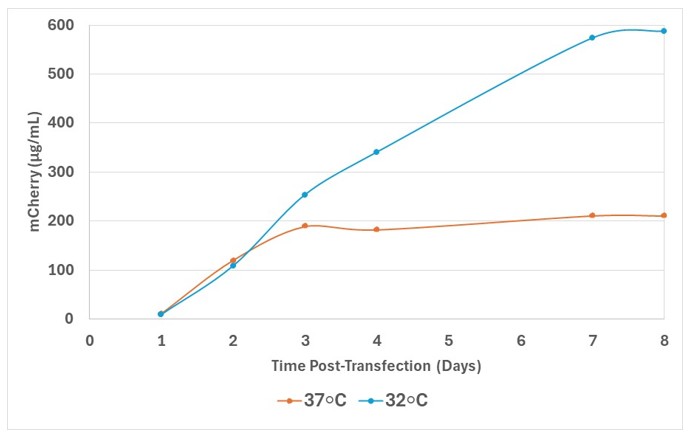
Figure 2: Comparison of intracellular mCherry expression in ExpiCHO-S cells cultured at 32oC or 37oC 24 hr post transfection.
Another method is the use of histone deacetylase inhibitors (HDACi) such as valproic acid (VPA) and sodium butyrate. In vivo and in vitro studies have demonstrated that transcription from extra genomic DNA decreases as a result of histone deacetylation and this contributes significantly to low recombinant protein yields for transient gene expression (Backliwal et. al., 2008). Addition of HDACi to cultures can supress this negative epigenetic effect and enhance expression (Figure 3).
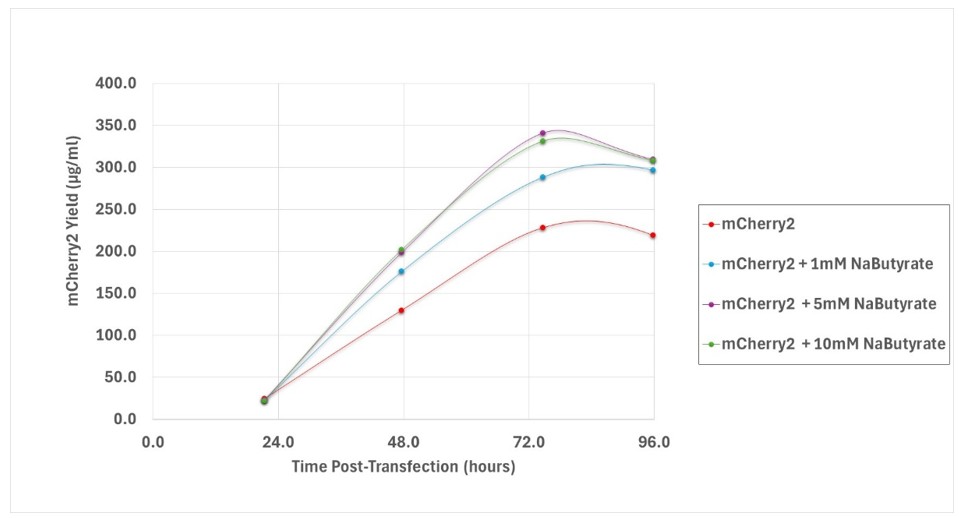
Figure 3: Expression of intracellular mCherry fluorescent protein in Expi293F cells supplemented with sodium butyrate at 1 mM, 5 mM and 10 mM.
Cells also respond to addition of HDACi with reduced growth rate and finally a decline of viability so careful adjustment of both the concentration and the timing of HDACi supplementation is required.
The addition of certain additives such as protein hydrolysates, ferric citrate, putrescine and trace elements can also improve recombinant protein yield (You et. al., 2013). In addition to this, combining additives which enhance protein production has been shown to have a complementary effect and further improve expression. Protein hydrolysates severely interfere with the process of cationic polymer-mediated transient transfection, so they should be added at a later timepoint, after transfection. As with the other approaches, it is important to identify the optimal timepoint, concentration and combinations for adding additives.
Combining the different methods for improving protein production can have a synergistic effect and lead to greater enhancement of transient expression. The use of a design of experiment (DoE) approach, can often be really helpful in defining the optimal conditions for maximum protein yield when several parameters need to be taken into account (Abbott et. al., 2015). A previous Case Study outlined a DoE study in HEK293-6E cells to investigate transfection DNA concentration, PEI:DNA ratio, cell density and feeding regimes, with cell viability and mCherry protein expression levels compared as the output for each condition tested (Figure 4).
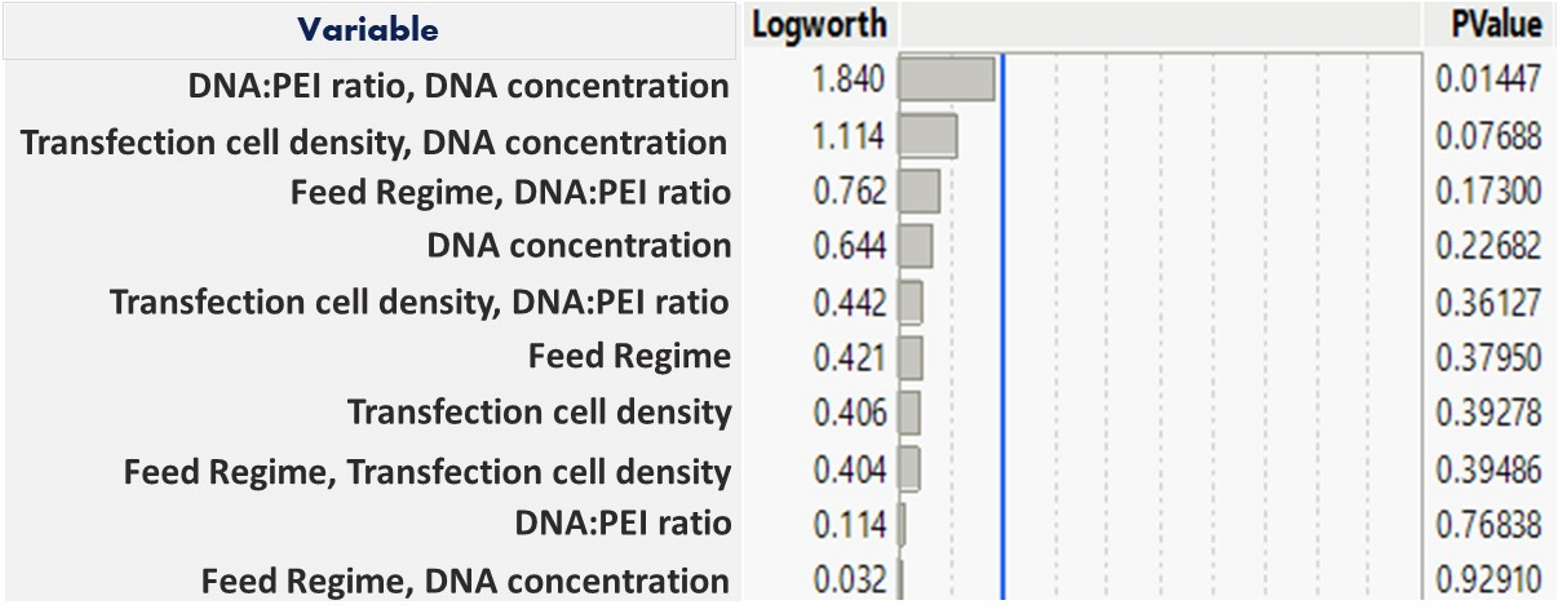
Figure 4: Logworth analysis of DoE data for mCherry yield in HEK293-6E cells.
Furthermore, we can do a direct comparison of expression of a ‘difficult to express protein’ across the different mammalian cell lines we offer at Sygnature Discovery and select the cell line giving maximum protein expression and protein purity for subsequent larger-scale expression. A recent BLOG described how expressing one such ‘difficult to express protein’ in CHO-3E7 cells led to a 10-fold higher expression than expression in HEK293-6E cells (figure 5).
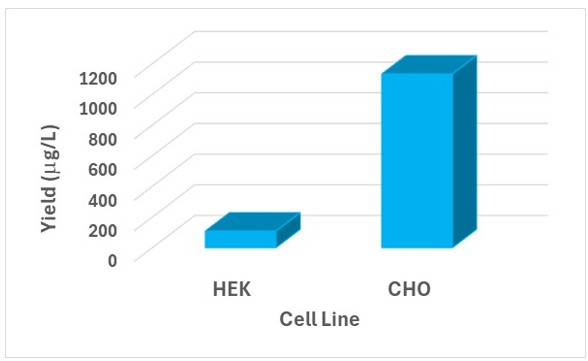
Figure 5: Protein yields for a ‘difficult to express protein’ from optimised HEK293-6E and CHO-3E7 transient transfection expression systems; 500 mL volume purified by a multi-step regime.
If you have a protein expression and purification project that you think would benefit not just from the approaches outlined here, but leveraging the extensive experience of our cell team, then please do not hesitate to get in touch with us via info@peakproteins.com.
References
Abbott WM, Middleton B, Kartberg F, Claesson J, Roth R, Fisher D. Optimisation of a simple method to transiently transfect a CHO cell line in high-throughput and at large scale. Protein Expr Purif. 2015 Dec;116:113-9. doi: 10.1016/j.pep.2015.08.016.
Backliwal G, Hildinger M, Kuettel I, Delegrange F, Hacker DL, Wurm FM. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol Bioeng. 2008 Sep 1;101(1):182-9. doi: 10.1002/bit.21882.
Estes B, Hsu YR, Tam LT, Sheng J, Stevens J, Haldankar R. Uncovering methods for the prevention of protein aggregation and improvement of product quality in a transient expression system. Biotechnol Prog. 2015 Jan-Feb;31(1):258-67. doi: 10.1002/btpr.2021.
Hussain H, Maldonado-Agurto R, Dickson AJ. The endoplasmic reticulum and unfolded protein response in the control of mammalian recombinant protein production. Biotechnol Lett. 2014 Aug;36(8):1581-93. doi: 10.1007/s10529-014-1537-y.
You M, Liu Y, Chen Y, Guo J, Wu J, Fu Y, Shen R, Qi R, Luo W, Xia N. Maximizing antibody production in suspension-cultured mammalian cells by the customized transient gene expression method. Biosci Biotechnol Biochem. 2013;77(6):1207-13. doi: 10.1271/bbb.120968.