Beginners Guide to Glycosylation of Proteins
What are post translational modifications?
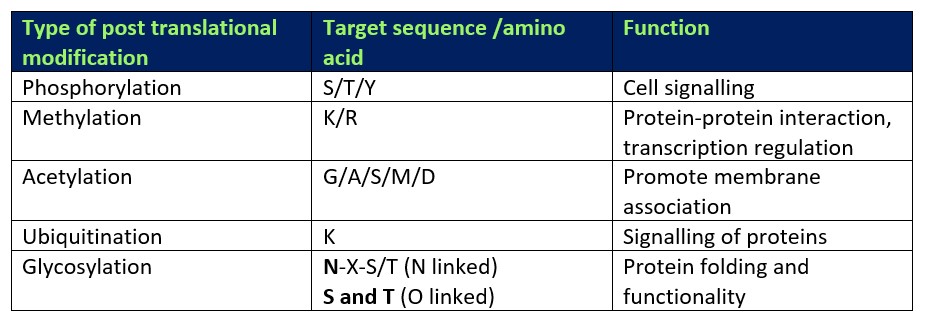
As a protein reaches the final stages of protein synthesis, it undergoes a series of post translational modifications (PTMs) which help define the biophysical characteristics of the final protein. PTMs can be specific to a single amino acid which is itself part of a certain target sequence of amino acids (see Table 1). To this day, it is apparent that there are numerous different modifications a single protein can obtain. These can include the addition of chemical groups such as phosphorylation, methylation or acetylation (Wang, Peterson and Loring, 2013). As well as the addition of chemical groups, some other forms of PTMs include the binding of small peptides such as ubiquitin which play important role in signalling pathways, especially for targeting proteins for proteasomal degradation and membrane trafficking (Schrader, Harstad and Matouschek, 2009; Komander and Rape, 2012). All of these modifications confer a vast array of functions, too many to be detailed in this specific blog. However, one of the more complex and important types of PTMs is glycosylation which will be the main focus of this blog.
Table 1. Types of PTM and their functions
What is glycosylation and why is it important for proteins?
Glycosylation is a common type of PTM where highly complex sugars or glycans are attached to numerous types of proteins. Modifications of this form generally occur after translation in the endoplasmic reticulum (ER) and the Golgi apparatus, through the highly organised activity of numerous enzymes. Numerous studies have found that glycosylation of proteins can occur both during and after protein folding, and in some cases, glycosylation mid folding can help the protein to fold correctly (Shental-Bechor & Levy, 2008).
Although there are many types of glycosylation, the two more prominent types are N- and O-linked glycosylation. N-linked glycosylation occurs specifically on the amide nitrogen atom of the amino acid asparagine (see Figure 1A) which is recognised as part of a glycosylation site N-X-S/T (where X can be any amino acid except proline). On the other hand, O-linked glycosylation is the attachment of glycans on the hydroxyl group oxygen atom (see Figure 1B) of either serine or threonine.
There is a vast diversity of N-linked glycans, however, the first residue, N-acetylglucosamine (GlcNAc), is constant and is followed by the sequential addition of other sugars such as mannose, galactose, fructose and finally topping off with sialic acid (see Figure 2A). This creates complex and highly branched glycan chains which can then provide numerous useful functions for glycoproteins, . The O-linked sugars can be relatively simple consisting of just 3-4 saccharide units based around the core (see Figure 2B). However, they can also be highly complex with critical roles in the extracellular matrix. Thus, for example proteoglycans are heavily modified with very long linear carbohydrate chains that are very negatively charged due to the presence of multiple sulphate groups and uronic acid.
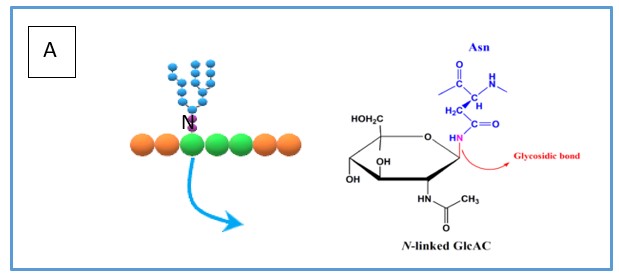
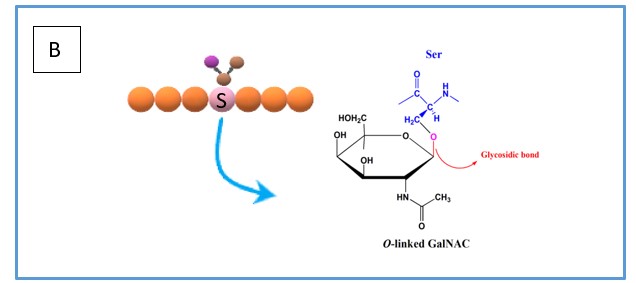
Figure 1. (A) simplified overview of how N-linked glycans are bonded to the asparagine residue including the chemical bond. (B) simplified overview of how O-linked glycans are bonded to the serine residue including the chemical bond.
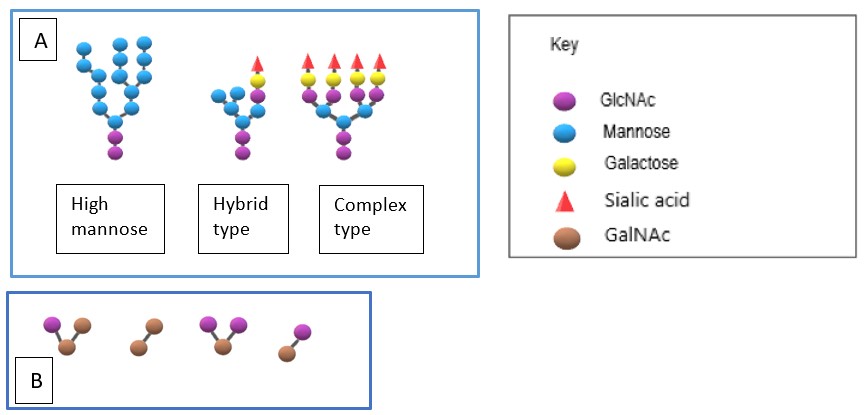
Figure 2. (A) The three different types of core glycans that N-linked glycosylation can exhibit, (B) The different types of core glycans that form the basis of O-linked glycosylation. These can then be modified with additional saccharides
Analysing glycosylated proteins at Peak Proteins
SDS-PAGE
At Peak Proteins we have a wealth of experience working with glycosylated proteins. We can predict glycosylation by looking for specific amino acid motifs and can also visualise glycosylation directly by looking at bands on an SDS-PAGE gel. Glycosylated proteins often show a characteristic smear on an SDS-PAGE gel due to the heterogeneity in the glycosylation, whereas non glycosylated proteins have a distinct singular band (see Figure 3).
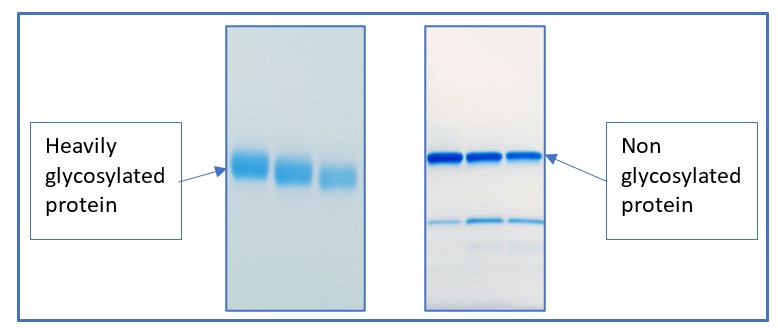
Figure 3. SDS-PAGE gels showing the difference between glycosylated proteins (left) and non-glycosylated proteins (right).
Mass spectrometry
Although SDS-PAGE gels are the easiest way to visualise glycosylation, it is not always the most accurate way to confirm glycosylation is present. At Peak Proteins we use a range of mass spectrometry analysis tools to fully investigate and confirm glycosylation in proteins. Intact mass spectrometry is useful to fully understand the heterogeneity of glycosylation as well as to profile the type of glycans present on a protein (see Figure 4).
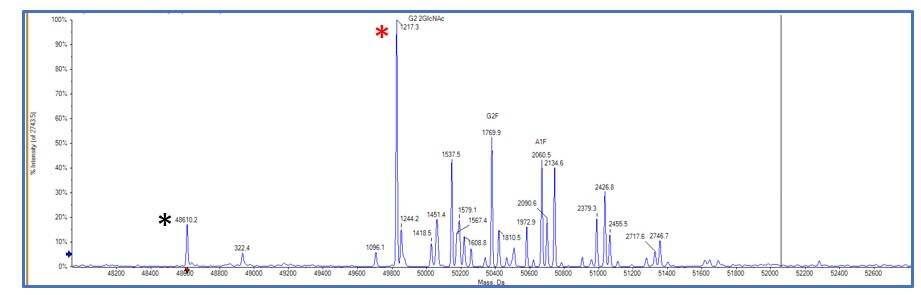
Figure 4. Intact mass spectrometry results showing the identity of the types of glycans present in a protein sample. Red asterisk corresponds to the identity of a glycan and its molecular weight compared to the unmodified protein mass marked with the black asterisk.
As well as using intact mass spec to identify specific glycans associated with a protein, another type of mass spec analysis known as peptide mapping is equally useful in identifying if a protein is glycosylated or not. Peptide mapping is a service we offer in order to obtain the identity of a specific protein from a band from an SDS-PAGE gel. After digesting the band with trypsin and/or chymotrypsin and running the sample on the mass spec, we can then identify the peptides present to compare with the full sequence of the protein. In cases where particular peptides cannot be detected and that encompass the N-X-S/T motif, this is suggestive that glycosylation is present. To further explain this, in one of our case studies, we used peptide mapping to reveal this heterogeneous nature of one of our proteins. This allowed us to purify and separate the protein into non-glycosylated and glycosylated forms.
Removal of N-linked glycosylation
Although it is known that glycosylation is can be important for the functionality of proteins, the flexibility and heterogeneity of these PTMs can cause issues for structural studies by preventing protein crystallisation. In such cases we can use a simple procedure that can remove this unwanted glycosylation. Treatment of cells with Kifunensine which is a mannosidase inhibitor and Endoglycosidase H which cleaves oligosaccharides which can result in proteins with solely the initial GlcNAc.
Having various analysis techniques to hand, such as intact mass spectrometry and peptide mapping, helps us to better understand the glycosylation of target proteins. Here at Peak Proteins, we have many examples of successful projects using these simple but effective techniques. To learn more about our approach to providing proteins to clients, you can read our case studies or contact us directly at info@peakproteins.com.
References:
- Komander, D. and Rape, M., 2012. The Ubiquitin Code. Annual Review of Biochemistry, 81(1), pp.203-229.
- Schrader, E., Harstad, K. and Matouschek, A., 2009. Targeting proteins for degradation. Nature Chemical Biology, 5(11), pp.815-822.
- Shental-Bechor, D., & Levy, Y. (2008). Effect of glycosylation on protein folding: A close look at thermodynamic stabilization. Proceedings of the National Academy of Sciences of the United States of America, 105(24), 8256–8261. https://doi.org/10.1073/pnas.0801340105


