How to Make Membrane Proteins and Not Unfold Them Q&A
Following our recent webcast “How to Make Membrane Proteins and not Unfold Them”, (produced by Peak Proteins and hosted by Nature Research Custom Media), Dr Steven Harborne and Dr Alice Rothnie answers some of the questions asked by attendees.
Where possible, the preference would always be to work with the native protein sequence. The SMALP technology is really important for this, as membrane proteins extracted in SMALPs are often significantly more stable than in detergent. Our suggestion would certainly be to use SMALPs first before trying any stabilising point mutations. However, in my experience there are some membrane proteins that SMALPs cannot stabilise. One possible reason may be that they are expressed in the membrane in a non-functional state to begin with, or they very rapidly unfold even in a recombinant membrane environment. If you do have a very difficult membrane protein like this then a logical next step would be to move to the stabilising point mutations, which may assist with folding, stability and expression. In this case, SMALPs could additionally be used in combination with a mutation stabilised protein. A final thing to consider and may preclude the use of SMALPs is when the protein is exclusively being directed for X-ray crystallography. Unfortunately, SMALPs are not compatible with crystallisation, so in this case the only option may be to stabilise the protein in a different way e.g stabilising point mutations.
At the moment, the IMPROvER analysis is limited by a number of factors at each stage. Firstly, in the data-driven approach, the dataset used to produce the scoring matrix was limited to a few GPCRs only, and therefore the predictions draw on a limited set of input information and may be slightly skewed toward GPCR predictions. This would be improved if there was a broader dataset that also encompassed other membrane proteins, and perhaps an AI/neural network approach could be applied. Secondly, in the deep-sequence module, the suggestions for advantageous mutations may not be limited to stabilisation and may affect other aspects of protein biochemistry such as protein expression, folding kinetics or catalytic activity, which were not taken into account. Finally, the model-based approach is limited by the quality of the homology models provided. Better input models would ensure the in-silico mutagenesis reflects the in-vivo case more accurately and provide better predictions. Furthermore, the in-silico melting used is based on very simple calculations that make assumptions about solvation, electrostatics and side chain pKa’s that may not reflect the in-vivo case well. Molecular dynamics simulations would provide a more accurate representation of these factors, and also allow a dynamic look at the protein rather than a static snapshot, however the computational overheads for such analyses would decrease the accessibility of this approach.
I think there will always be a gap between the ease of working with soluble proteins versus the challenges of work on membrane proteins. However, there are a number of emerging tools and technologies that are helping us to tackle ever more challenging membrane proteins. The SMALP technology is one great example. There have also been advances in detergents that are available, such as the neopentyl glycol class. These gentle detergents provide even more gentle methods to extract membrane proteins, including in complex with native lipids.
SMALP technology is really the first iteration of a larger effort to use a greater number of co-polymers that self-assemble into lipid discs with different properties. The original SMALP formulations vary to some degree in the ratios of the styrene to maleic acid moieties, and in their average length and dispersity depending on how they have been manufactured. Quite a lot of work has been done to try and investigate what effect these different factors have on the ability to solubilise and stabilise membrane proteins. Moving toward more homogeneous polymer synthesis with well-defined styrene to maleic acid ratios appears to be one way to improve the desirable properties. Furthermore, effort is being made to overcome some of the shortcomings of the original SMA co-polymer, for example sensitivity to low pH, sensitivity to divalent cations and interference of the polymer with affinity tag binding to purification resin. Derivatisations of the SMA polymer as well as some novel polymers are all being investigated for these purposes. Finally, the polymers can be derivatised with other useful functional groups. For example, fluorescent tags or affinity tags (e.g. biotin) can be added directly to the polymer rather than the protein. All these advances will provide a larger toolkit for studying membrane proteins, and I think there will be lots of exciting applications across different fields that will utilise these developments. For more information, the SMALP Network is a great resource.
In short, it is likely that any stabilisation locks the protein into a single state either at a single step in the enzymatic cycle or an abortive low energy state not found often in-vivo, and this is why it works. Membrane proteins such as GPCRs and transporters are very dynamic and flexible by their nature as this is what allows them to carry out a biological function in the cell, but also makes them unstable to work with. Stabilisation of the membrane protein, particularly for X-ray crystallography aims to reduce the inherent flexibility and lock the protein in a defined state that can be crystallised. Thus, the state that is observed is often a single abortive state pulled out of the continuum of possible states visited by a membrane protein during its biological function. However, to begin understanding the protein from structure, we must start somewhere, and it is often not possible to crystallise or even purify non-stabilised versions of the protein!
This is a very interesting question, and one that could be looked at in much greater depth than is possible here. In short, I think it is a slight overstatement to say that AlphaFold has completely removed the need to solve protein structures experimentally. There is no doubt that AlphaFold did an excellent job at predicting the shape of proteins to a better degree than the competing groups at the recent CASP meeting. However, of the test set of proteins that was used in that evaluation, there was only one membrane protein example and this was one of the targets on which AlphaFold performed fairly poorly. So, for membrane proteins I think there is still a little way to go. I’m still excited about it though, and I think having more accurate models can only help research. Understanding a single structure of a protein is rarely the final end point on its own. Even if that structure was arrived at by X-ray crystallography or Cryo-EM, we would always want to extract some further information about the target, be it something about the physiological mechanism of the protein or be something about ligand or drug binding properties. I don’t think we’re ever going to remove the need to experimentally have a look at some of these aspects, and I think for the foreseeable future, any prediction that is made in-silico will need to be backed-up experimentally, particularly for membrane proteins. For a more in-depth look at this topic, and how it relates to the work that Peak Proteins does please take a look at one of our recent blog: Have Robots Taken My Job?.
All the individual modules of IMPROvER originated from methodology that is already well established for soluble proteins and many other tools already exist for this, but their use on membrane proteins has always been limited as membrane protein have an extra level of inherent difficulty associated. The combinatorial approach of IMPROvER drawing on many different in-silico methods is one of the key things that helps to increase the applicability to membrane proteins. Furthermore, there is more need to stabilise membrane proteins as a matter of course in their study as they are inherently so tricky to work with. To my knowledge, such a combinatorial approach has not been applied to soluble proteins, but there would be nothing to prevent such an analysis.
Tm is usually calculated from melting curves, either as the peak in a first derivative of the melting curve or the midpoint in a 4-parameter dose-response curve: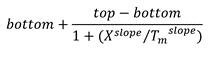
More information about thermal-melting assays can be found in our blog: Thermofluor Stability Assays: More Than Just Thermal Melting.
Sadly, it is not a simple answer. One of the challenges is that the SMA is sensitive to divalent cations such as magnesium and ATPase activity requires magnesium, meaning they are incompatible with one another and activity cannot be confirmed this way. A further complication even if the divalent cation issue was overcome, it is possibly the SMA holds the protein a little too tight for some conformational changes in the ABC transporter mechanistic cycle on ATP hydrolysis. This could be seen as an advantage however, as we may be able to trap different states before solubilising, and the transporter will stay trapped in those particular states. A useful tool if you want to study those specific states. It should be said though, that the alternative polymer DIBMA is also available, which makes bigger discs with more fluid lipids within them and is not so sensitive to divalent cations. This is work that is ongoing, but hopefully that will be the answer to address ATPase activity of ABC transporters. It should be noted that in the field in general, there are many examples of membrane proteins that are not ABC transporters that have been shown to retain wild-type activity in SMALPs including transporters and GPCRs.
In my experience, if you’ve got the polymers freeze-dried you can keep them for very extensive lengths of time. If they’re in the liquid format, recommended storage is at 4˚C, and I would say they usually last more than one month. For long term storage I would try freeze drying, and if you don’t have facilities for that yourself there are companies that sell them in a freeze-dried format.
In our hands Peak Proteins ELRIC Feb 2021 Poster, comparing detergent solubilised and SMA solubilised GPCRs, there was a very clear advantage in using SMALPs. The GPCRs were stable at both 4˚C and 20˚C for a longer period of time when in SMALPs when compared to solubilisation in DDM. We did not test freeze/thaw in this case, but from the wider field, it has been found that SMA encapsulated membrane proteins are far more resistant to harsher conditions including freeze/thawing and even freeze-drying.
I think one of the original definitions of lipid rafts was because they weren’t accessible to detergent solubilisation. In my experience, SMA is capable of solubilising all different lipid components. The kinetics vary depending on the lipid composition though, so perhaps the lipid components found in rafts solubilise more slowly. I do believe they have been all shown to solubilise by Antoinette Killian’s lab in the Netherlands who have done a lot of work on this.
Yes, I think this can absolutely be the case. When we study membrane proteins, particularly in relation to their physiological function, the ideal system for study would be one that is most like the native membrane environment. Different methods of extraction and formulation provide different levels of similarity to the native environment. Detergent solubilisation tends to be the most unlike the native environment as the majority of the lipids are stripped away, but even then, using the gentlest detergents often helps retain tightly bound lipids, and the proteins can retain function. Aside from detergent solubilised protein, the protein can be reconstituted into liposomes, bicelles, nanodiscs or amphipoles that provide different levels of similarity to a membrane environment. However, in each of these cases the native lipids are stripped away, and then a reconstituted system is added back. This makes the system more controllable and reproducible but abstracts it away from the native case. SMALPs on the other hand extract the membrane protein directly from the native membrane, and so it could be argued this would represent the most native composition of the lipids encapsulated with the protein. In terms of how all these different systems impact the functional dynamics of membrane proteins, I think this is still ongoing work in the field. How the different encapsulations affect mechanism will also vary on a case-by-case basis, as some membrane proteins are more or less influenced by lipid composition and membrane topology.
Possibly. One of the things with DIBMA is that it makes bigger discs so it is plausible that more than one protein is isolated in the same disc. We may be finding proteins that associate with the target protein, but this is not yet fully resolved.
With the polymers you can extract from either. We routinely use the membrane fractions as DNA contamination is greatly reduced without the need to add DNAase. However, it is feasible to do it from whole cells too, and other groups do this. There is an excellent Smith, A.J., Wright, K.E., Muench, S.P. et al. Styrene maleic acid recovers proteins from mammalian cells and tissues while avoiding significant cell death. Sci Rep 9, 16408 (2019) where they add very small amounts of SMA to live cells. They were able to extract small amounts of membrane from the live cells without killing them.
Typically, between 1-2% w/v of SMA is used for solubilisation, and the solubilisation must be carried out at room temperature rather than 4˚C for 1-2 hours. For affinity purification steps following solubilisation, it is a good idea to dilute the sample so that the polymer concentration is lower than 0.5%, as the polymer can interfere with efficient column binding.
Yes, is the short answer. Sophie J. Hesketh, David P. Klebl, Anna J. Higgins, Maren Thomsen, Isabelle B. Pickles, Frank Sobott, Asipu Sivaprasadarao, Vincent L.G. Postis, Stephen P. Muench, Styrene maleic-acid lipid particles (SMALPs) into detergent or amphipols: An exchange protocol for membrane protein characterisation, Biochimica et Biophysica Acta (BBA) – Biomembranes, Volume 1862, Issue 5, 2020 have done this, and we have also tested this a little too. You can incubate your SMALP purified protein with liposomes and add some magnesium. The SMA will dissociate and hopefully the protein will assemble into the liposome. You can also swap the SMA for detergent or amphipols in a similar manner if the downstream technique is not compatible with SMA (e.g. for crystallography).
In each of the test cases from the IMPROvER publication we could demonstrate the majority of the mutations did not adversely affect ligand binding or activity of the proteins. Designed into the analysis is the ability to protect sites from suggested mutation if they are already known to be involved with ligand binding or protein mechanism. Thus, the risk of unduly inactivating protein ligand binding properties is reduced.
In short, this is not really what IMPROvER was designed to assist with. Mutations suggested by IMPROvER are likely stabilising because they help increase the trans-membrane helix-helix interactions in particularly unstable regions of a transmembrane protein. Making mutations in a single transmembrane helix pass is unlikely to have this desired effect. However, in this case trying different detergents or even trying SMA solubilisation would be a preferable route to achieving stabilisation.
For extraction of membrane proteins from E. coli it is likely that a membrane isolation prior to purification would be preferable. There are a number of different techniques to do this, and the inner and outer membranes can even be separated. Sonication is likely to fragment the membranes too much and there is also a risk undue heat will be transferred into the sample, and membrane preparation is more difficult in this case. Instead, a French press or similar high-pressure device tends to be preferable. Following lysis, differential centrifugation is used to sperate heavy insoluble material from the cell from the cytoplasmic material and the membranes. Membranes can be harvested using very high-speed centrifugation, usually in an ultracentrifuge.


