Mutations, Mutations, Mutations
The mutations seen in the COVID-19 Variants of Concern
The coronavirus disease-19 (COVID-19) is a respiratory disease responsible for the current worldwide pandemic. It is caused by the SARS-CoV-2 virus which is an enveloped, positive-strand RNA virus. SARS-CoV-2 uses the spike glycoprotein (located on its envelope) to enter its target cells via the human angiotensin-converting enzyme 2 (ACE2) receptor. The spike protein itself contains two functional subunits (S1 and S2) with a furin cleavage site at the boundary between the two subunits. The S1 subunit contains the receptor-binding domain (RBD) and is responsible for binding to the ACE2 receptor, while the S2 subunit is responsible for the fusion of the viral and cellular membranes. The RBD can be found in two conformations: the ‘up’ conformation, which is receptor accessible, or the ‘down’ conformation’ which is receptor shielded.
Although SARS-CoV-2 is an RNA virus, it can proofread its RNA duplication and so has a lower rate of mutations than other RNA viruses. That being said, most variability in mutations of the spike protein can be found on the RBD and mutations do occur. As a result of these mutations new variants of SARS-CoV-2 have cropped up throughout the pandemic. Of these, four have been classed as variants of concern (VOI) due to their potential increase in transmissibility, increase in virulence, or ability to escape current vaccines. The pango lineage is generally used to refer to these variants in scientific publications but colloquially, these have been named after the places where the variant was first found and characterised. Due to fears of discrimination by the general public, however, the WHO has given them new labels based on the Greek alphabet.
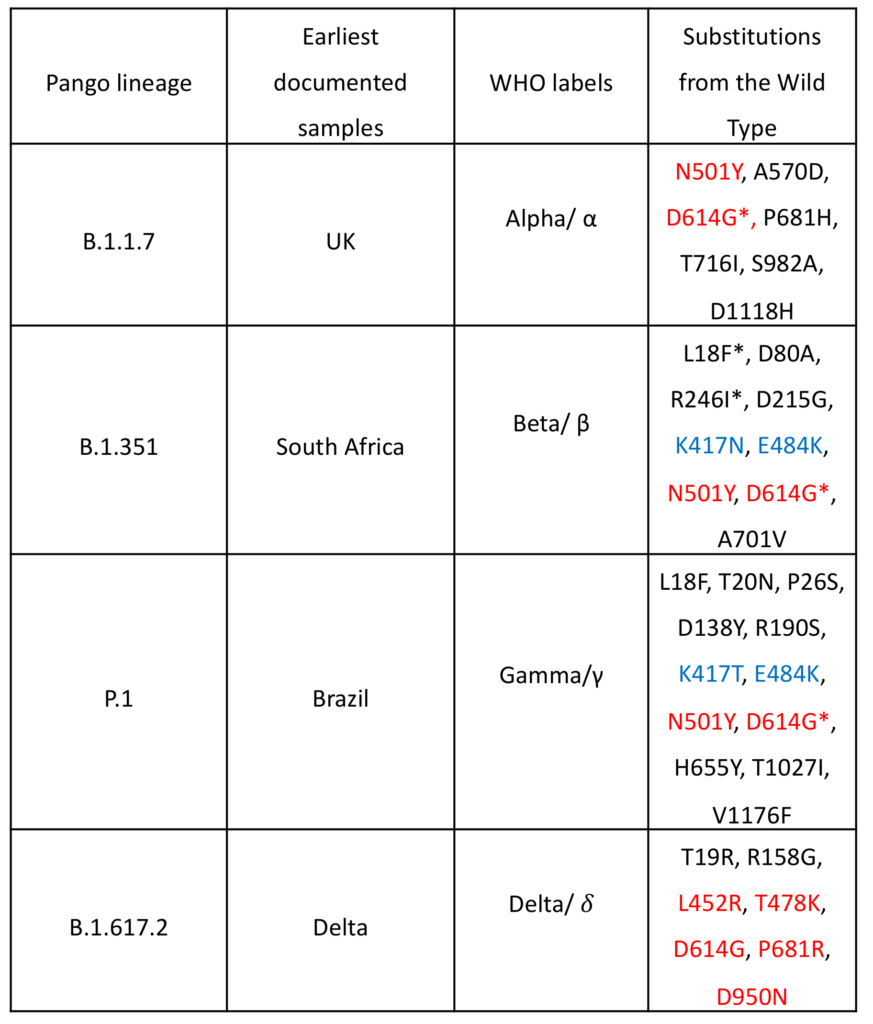
Table 1: COVID-19 Variants of Concern; mutations in red are associated with increased transmissibility and mutations in blue are considered escape mutations (note: N501Y can also be considered an escape mutation but is mainly involved in increased transmissibility); *indicates a mutation with high diversity within the lineage, or that the mutation was seen in the population where the variant first emerged before the variant emerged.
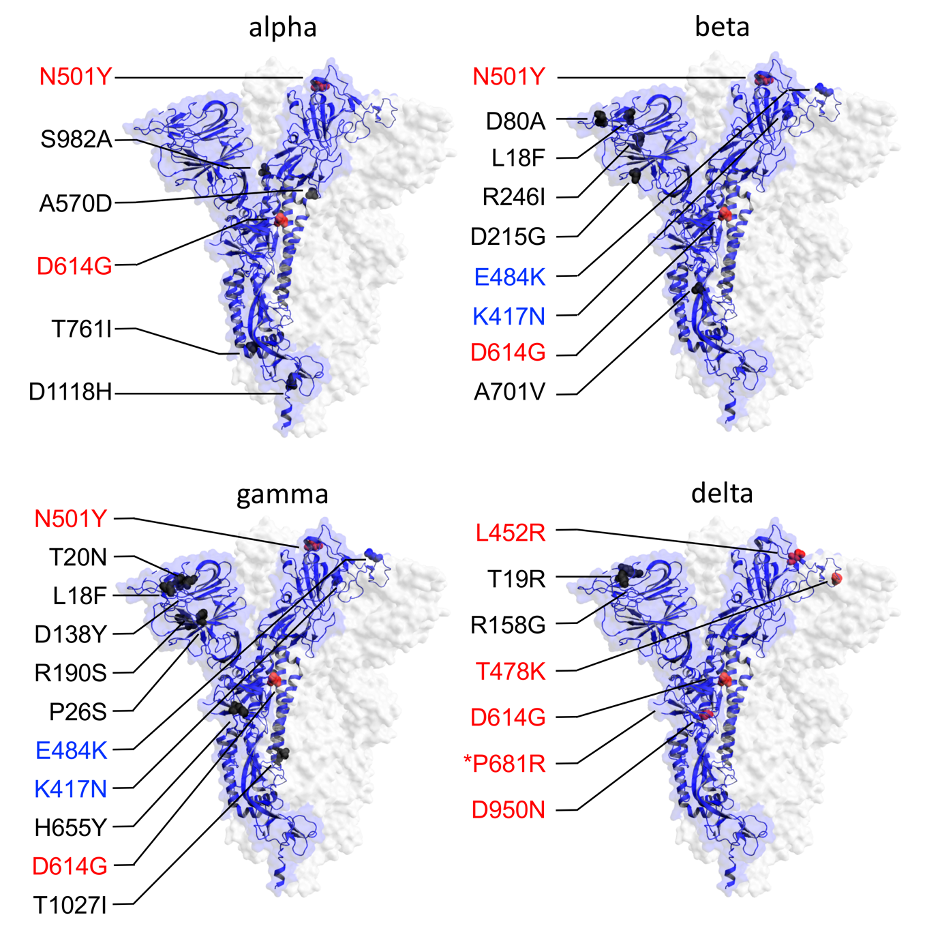 Figure 1: COVID-19 Variants of Concern SPIKE protein, showing mutations and highlighting mutations which cause increased transmissibility (red) and escape mutations (blue) as per Table 1.
Figure 1: COVID-19 Variants of Concern SPIKE protein, showing mutations and highlighting mutations which cause increased transmissibility (red) and escape mutations (blue) as per Table 1.
Increased transmissibility
The first of these variants to be characterised was the alpha variant. This variant showed increased transmissibility, believed to be caused by the N501Y mutation. N501Y is one of the six key ACE2 contact residues found on the RBD. In the wild-type, N501 has two non-bonded contacts to the ACE2 K353 and Y41 residues. In the alpha variant, N501Y has a hydrogen bond to K353 and non-bonded contacts to D38 and Y41. Asparagine is a polar amino acid while tyrosine is an aromatic amino acid with an -OH group to act as a strong donor-acceptor for a hydrogen bond. This aromaticity also allows for an increase in – interactions. Tyrosine is also much larger than asparagine, and so the mutation causes conformational changes that mean that non-mutant border residues such as Y500, G501, V503 and Y505 show polar interactions with the ACE2 receptor in the alpha variant. This increased affinity for the ACE2 receptor leads to stronger binding between the spike protein and the ACE2 receptor, therefore increasing the transmissibility of the alpha variant when compared to the wild-type SARS-CoV-2. The beta and gamma variants also show this N501Y mutation and the corresponding increased transmissibility.
The delta variant is the latest of the four to be characterised. Although it does not contain the N501Y mutation, it also shows increased transmissibility. The main cause of this is likely the L452R mutation. While L452 is not directly located on the RBD binding site, its location is close to a negatively charged patch on the ACE2 receptor (E35, E37 and D38). L452 (along with F490 and L492) forms a hydrophobic patch on the spike RBD. By changing from a hydrophobic leucine to a positively charged arginine, the interaction between the RBD and the ACE2 negatively charged patch is stronger and this stronger interaction promotes binding between the SARS-CoV-2 spike protein and the human ACE2 receptor.
The delta variant also contains the T478K mutation. This mutation is also not found in the three other variants. It is believed that the inherently long side-chain of the lysine compared to the threonine reduces the distance between the spike protein and the ACE2 receptor. In the original variant, there were only 10 ACE2 residues in close proximity to T478 and L452, while in the delta variant there are 24 ACE2 residues in close proximity to T478K and L452R. This increased affinity between the spike protein and the ACE2 receptor also increases transmissibility. Furthermore, although the exact mechanisms have not been found yet, it is believed that the P681R and D950N mutations, due to their proximity to the S1/S2 cleavage site, lead to conformational changes which result in the RBD opening. In the same way, the D614G mutation causes conformational changes which favour the ‘up’ RBD conformation, while is required for spike protein and ACE2 receptor binding. The D614G mutation was seen in all four variants.
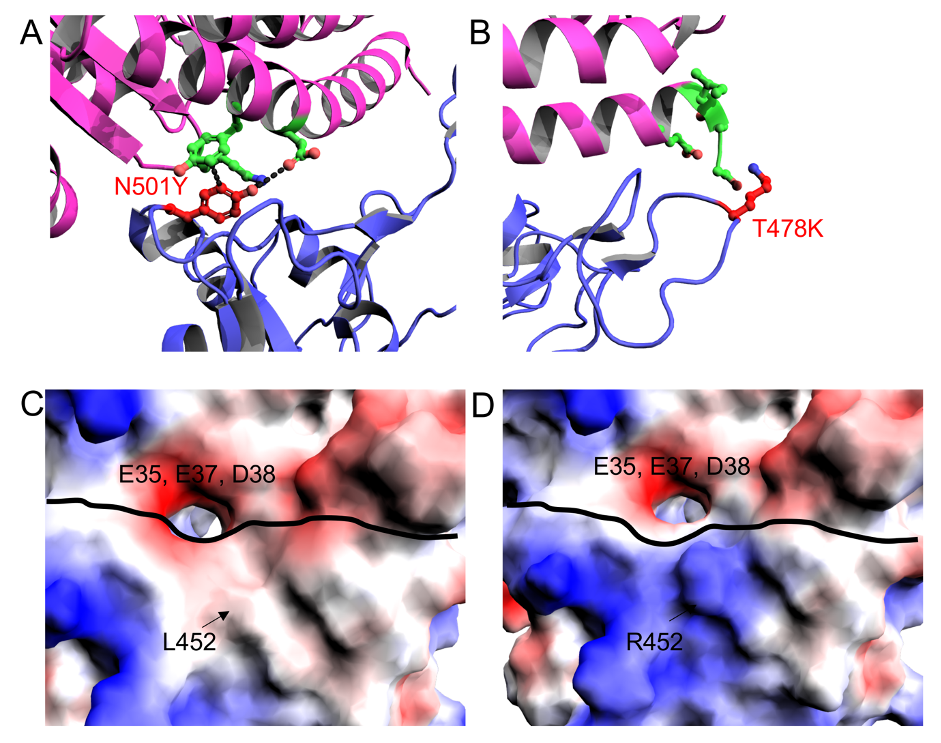 Figure 2: A) N501Y substitution and the resulting hydrogen bonds between the SPIKE protein and ACE2 receptor, B) T478K substitution and the resulting reduced gap between the SPIKE protein and ACE2 receptor, C) L452 residue on SPIKE protein forming part of the hydrophobic patch, in close proximity to the charged E35, E37 and D38, D) mutant R452 residue on SPIKE protein, now positively charged, which forms a stronger interaction between the SPIKE protein and the ACE2 receptor as a result of the negatively charged residues, E35, E37 and D38, on the ACE2 receptor
Figure 2: A) N501Y substitution and the resulting hydrogen bonds between the SPIKE protein and ACE2 receptor, B) T478K substitution and the resulting reduced gap between the SPIKE protein and ACE2 receptor, C) L452 residue on SPIKE protein forming part of the hydrophobic patch, in close proximity to the charged E35, E37 and D38, D) mutant R452 residue on SPIKE protein, now positively charged, which forms a stronger interaction between the SPIKE protein and the ACE2 receptor as a result of the negatively charged residues, E35, E37 and D38, on the ACE2 receptor
Escape mutations
Escape mutations are mutations that could potentially prevent antibody and SARS-CoV-2 binding, and/or reduce the efficacy of the current vaccines produced. In general, these escape mutations can be found in three discrete regions of the RBD. The first is the receptor-binding ridge within the receptor-binding motif and the second is a loop in the receptor-binding motif opposite the ridge (sites 443-450, and the structurally adjacent 494-501 residues). The third is a surface patch in the core RBD.
The antibodies themselves are classed based on their target region of the RBD. Class 1 antibodies are the type most frequently elicited during COVID-19 and include a public antibody response to the spike protein with the RBD ‘up’ conformation. Class 2 antibodies bind to both the ‘up’ and ‘down’ RBD conformations. Furthermore, most spike-based vaccines are designed to contain the RBD. Some studies classed this as residues 319-514, while others included residues 301-430.
One way to test for escape mutations is to use plasma and antibodies taken from people who have previously been infected with SARS-CoV-2 and test them against spike proteins containing the mutations. In one study, E484 was mutated to lysine (found in the beta and gamma variants), glutamine or proline. In the plasma from one subject, there was a 35-fold to 115-fold decrease in the neutralisation titre of the plasma. In another subject, the E484K mutation reduced the neutralisation titre of the plasma 10-fold. Although the neutralisation wasn’t as strong with the other subjects, mutations at E484 did generally cause the largest drops in neutralisation. In the same study, it was shown that K417N (found in the beta variant), did escape neutralisation by some monoclonal antibodies, but this effect was only seen weakly in some samples.
In another study, class 1 antibodies were tested and it was found that these were mainly centred around the K417 residue. The antibodies were in contact with 60-100% of the K417 side-chain-accessible surface. Class 2 antibodies were centred around the E484 residue, and were in contact with 40-100% side-chain-accessible surface. These contacts involved hydrogen bonds and charged interactions. When the beta variant itself was tested, it could not be neutralised by the class 2 antibodies, likely due to the E484K mutation. When a K417N/E484K/N501Y mutant was tested, 27% of the samples lost all neutralisation activity against it and only 23% of the samples were highly effective against the mutant.
However, another study showed that although individual classes of antibodies showed less potency against E484K, K417N or N501Y mutants, combinations of antibodies were effective against all the variants tested, which included K417N/E484K/N501Y mutants. Different studies also showed that non-neutralising antibodies, which do not interfere with the viral infectivity but are important in flagging the virus for immune cells like macrophages, were only minorly affected by these mutations if at all.
The four variants of concern that have emerged have been classed as such because of their increased transmissibility and/or their potential to escape current vaccinations. The alpha and delta variants mainly contained increased transmissibility mutations while the beta and gamma variants contained both increased transmissibility and escape mutations. The escape mutations are of particular concern as they have shown decreased binding to antibodies produced by people previously infected with SARS-CoV-2. These antibodies are likely to be those induced by the current vaccines as they were made using the spike proteins from the original SARS-CoV-2 strain. However, while different classes of antibodies were nullified by different escape mutations, combinations of the different classes of antibodies neutralised all the mutant variants. Therefore, while the escape mutations are a concern, the variants of concern can still be neutralised by the combination of different classes of neutralising and non-neutralising antibodies produced by the immune system upon infection or vaccination.
If you need ACE2 or Spike proteins, please have a look at our website for details or contact us at info@peakproteins.com


