In vivo biotinylation of a secreted protein from mammalian cells
Biotinylation of proteins is a powerful tool for protein science and our recent case study on the in vivo biotinylation of intracellular proteins by co-expression with BirA ligase described how this method is now used routinely at Peak Proteins.
A number of our mammalian expressed proteins, however, are secreted proteins and BirA is an intracellular protein. A method for biotinylation within the secretory pathway was reported in 2008. This method involved the co-expression of BirA and the target protein from a bicistronic plasmid, with BirA being fused to the signal leader peptide from a mouse immunoglobulin heavy chain; the idea being that both the target protein and BirA translocate to the endoplasmic reticulum (ER) where biotinylation occurs.
To provide further options for our clients’ projects, we decided to try a similar approach in our proprietory HEK293-6E cell expression system. We fused BirA to an IgK secretary leader signal in a separate plasmid for co-transfection and expression with the target protein. This trans approach was adopted to allow us to produce both non-biotinylated and biotinylated forms of the protein, if needed.
We chose a heterodimeric, secreted cytokine protein we have previously expressed and biotinylated successfully in vitro, as a case study protein to test this approach. One of the proteins was tagged with the short AviTag required for site specific biotinylation.
Co-transfection and expression
HEK293 cells were co-transfected with three plasmids expressing proteins with secretory signal signals: the two target heterodimeric proteins (α + β) and BirA. The BirA expression plasmid was transfected at a 10-fold lower level than the two target plasmids and cells were grown, supplemented with biotin. Expression was also performed without BirA present.
Purification
Cells were spun down and the supernatant was purified by nickel chelate affinity chromatography, showing two clear bands in the nickel eluate.
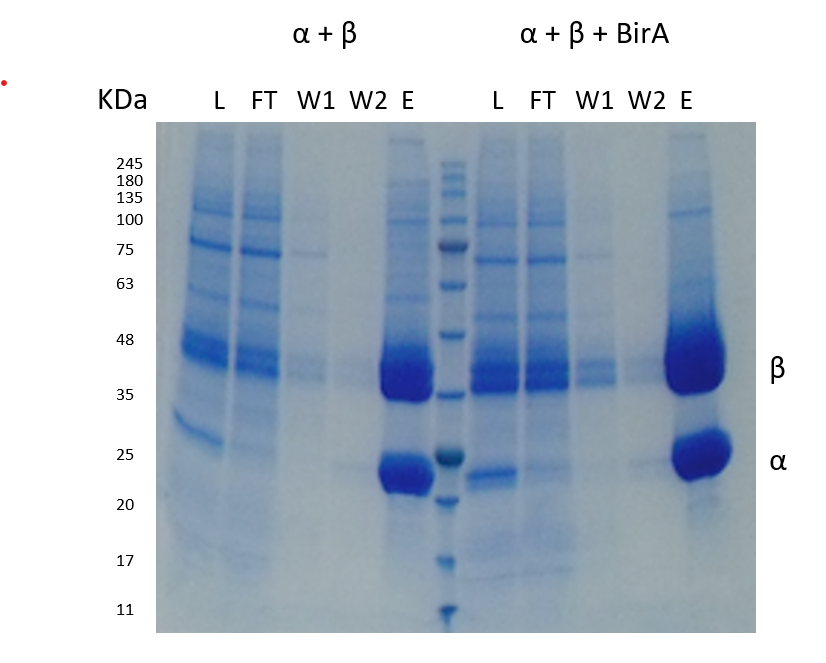
Intact Mass Analysis
Protein samples were loaded onto a Sciex X500B mass spectrometer, to obtain an intact mass value. This is something we do routinely as part of our QC for clients but also offer it as a separate mass spectrometry service for clients.
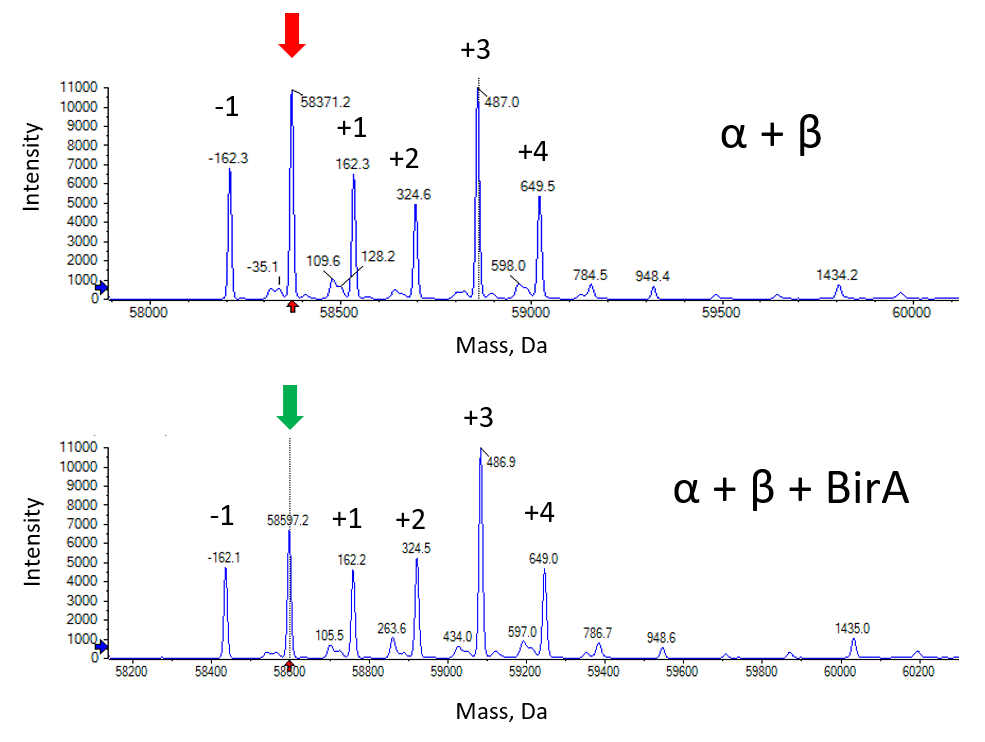
The combined size was predicted to be 57,004 based on the mass of proteins α and β but was anticipated to be higher due to additional glycosylation mass The main species in both conditions is highlighted above with an arrow and other species are relative to this species. These other species are various additions of multiples of +/- 162 Da, attributed to hexose, a common glycosylation in mammalian cells systems.
The main species was 226 Da larger when expressed with BirA. This size increase correlates with biotinylation of a single lysine – the site-specific residue in the AviTag. Furthermore, the shift in size was 100%, indicating we had achieved complete biotinylation of all the expressed protein.
Conclusion
This secreted in vivo biotinylation strategy was shown to be very effective and will provide another tool for our protein expression and purification service. The published strategy was shown to be successful for secretory and membrane proteins and with both N- and C-terminal tags suggesting this could be widely applied.


