Biotinylation of Recombinant Proteins
What is Biotin?
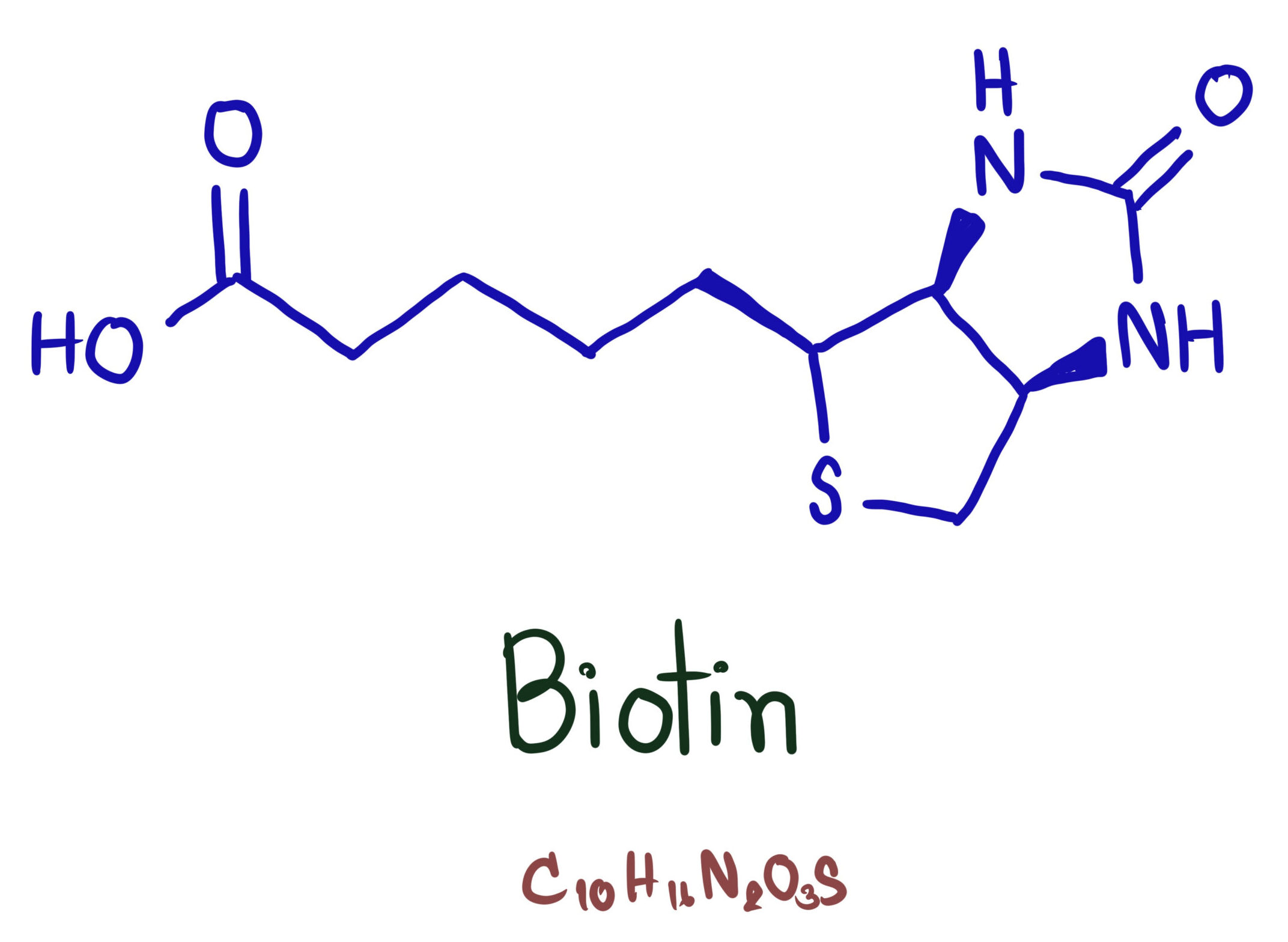
Biotin (or vitamin B7) is an essential coenzyme that is required by all organisms for life. Indeed, biotin’s name literally means ‘life giving’. Biotin is covalently linked near the active sites of four major classes of carboxylase enzyme with a variety of metabolic roles. In most bacteria, plants, and fungi, biotin is synthesised by the organism, but in higher eukaryotes, biotin is sourced externally. Humans get biotin from a small number of foods as well as from gut bacteria.
In cells, biotin is added to proteins through enzymatic biotinylation, a process carried out by biotin protein ligases (BPLs). BPLs are enzymes of extraordinary specificity with most organisms having fewer than five enzymes that are biotinylated. For example, BirA, the BPL of E. coli, biotinylates only a single cellular protein; the BCCP subunit of acetyl-CoA carboxylase.
The Discovery of Avidin and Streptavidin
Avidin was discovered Esmond Emerson Snell after the observation that chicks fed on egg white were suffering from biotin deficiency. This was discovered to be due to an egg white glycoprotein sequestering biotin which was named Avidin1,2. Avidin is now known to be produced by the oviducts of birds, reptiles, and amphibians. The exact purpose is unknown, but it is thought to inhibit bacterial growth by sequestering biotin. Streptavidin is a related protein discovered in the 1960s from the bacterium Streptomyces avidinii3, where it is also believed to act as an antibiotic factor. The key difference between these proteins is that unlike avidin, streptavidin and other bacterial derived avidins are non-glycoproteins.
The Incredibly Strong Biotin-Avidin Interaction
The biotin-avidin interactions have evolved to be one of the strongest known non-covalent interactions in nature, with a Kd in the range of 10-14 to 10-15 M (picomolar to femtomolar range). The bond is resistant to denaturants, extreme pH, extreme temperature, and detergents. Both streptavidin and avidin bind with high affinity, however, avidin has a higher degree of non-specificity due to its higher pI and glycan modifications.
Using Biotinylation in Protein Biology
The strength of the biotin-avidin interaction makes it an attractive molecular tool. Biotin is small and unlikely to disturb the natural function of a molecule. Biotin can be added to molecules enzymatically or chemically to proteins and nucleic acids. In protein biology, biotinylation can be used for highly sensitive protein detection. Biotinylation can also be used for protein isolation, with the interaction strength giving stable immobilisation and allowing for strong wash conditions. This makes biotinylation a great tool for protein purification, HTS or biophysics.
Biotinylation of AviTag Using BirA
Proteins can be specifically biotinylated using the natural specificity of the E. coli protein, BirA. BirA specifically biotinylates a single lysine (K122) on the BCCP protein4. Originally, BCCP was fused to proteins, but through peptide screening, a minimal peptide sequence was identified with a similar affinity to the natural substrate5. This was then further optimised to have an even higher affinity than BCCP6. Adding this AviTag peptide sequence to a protein allows specific biotinylation using recombinant BirA. We regularly use this technology at Peak Proteins to biotinylate proteins for our clients for a range of uses and also in collaboration with our parent company Sygnature Discovery for compound screening and SPR.
In vivo Biotinylation of Recombinant Proteins
Biotinylation of recombinant proteins can also be carried out in vivo by co-expressing the target protein along with BirA. This can be done in all expression systems we offer at Peak Proteins; E. coli7, insect cells8, and mammalian cells9. We have been using in vivo biotinylation a lot recently at Peak Proteins because it can offer several advantages for our clients.
- Reduced handling time preventing loss of difficult to work with proteins
- Reduced processing times decreases costs
- More efficient biotinylation with the option to biotinylation in vivo and in vitro
- Reduced chance of BirA contamination
For an example of how we used in vivo biotinylation in insect cells for a client, please read out recent case study.
References
- Kresge, N., Simoni, R. D. & Hill, R. L. The Discovery of Avidin by Esmond E. Snell. Journal of Biological Chemistry 279, e5–e6 (2004).
- Snell, E. E., Eakin, R. E. & Williams, R. J. A Quantitative Test for Biotin and Observations Regarding its Occurrence and Properties. J Am Chem Soc 62, 175–178 (1940).
- Tausig, F. & Wolf, F. J. Streptavidin—A substance with avidin-like properties produced by microorganisms. Biochem Biophys Res Commun 14, 205–209 (1964).
- Cronan, J. E. Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. 265, 10327–10333 (1990).
- Schatz, P. J. Use of Peptide Libraries to Map the Substrate Specificity of a Peptide-Modifying Enzyme: A 13 Residue Consensus Peptide Specifies Biotinylation in Escherichia coli. Bio/Technology 1993 11:10 11, 1138–1143 (1993).
- Beckett, D., Kovaleva, E. & Schatz, P. J. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci 8, 921 (1999).
- Gräslund, S., Savitsky, P. & Müller-Knapp, S. In Vivo Biotinylation of Antigens in E. coli. Methods Mol Biol 1586, 337–344 (2017).
- Duffy, S., Tsao, K. L. & Waugh, D. S. Site-specific, enzymatic biotinylation of recombinant proteins in Spodoptera frugiperda cells using biotin acceptor peptides. Anal Biochem 262, 122–128 (1998).
- Ioannou, M., Papageorgiou, D. N., Ogryzko, V. & Strouboulis, J. Mammalian expression vectors for metabolic biotinylation tandem affinity tagging by co-expression in cis of a mammalian codon-optimized BirA biotin ligase. BMC Res Notes 11, 390 (2018).
- Predonzani, A., Arnoldi, F., López-Requena, A. & Burrone, O. R. In vivo site-specific biotinylation of proteins within the secretory pathway using a single vector system. BMC Biotechnol 8, 41 (2008).

