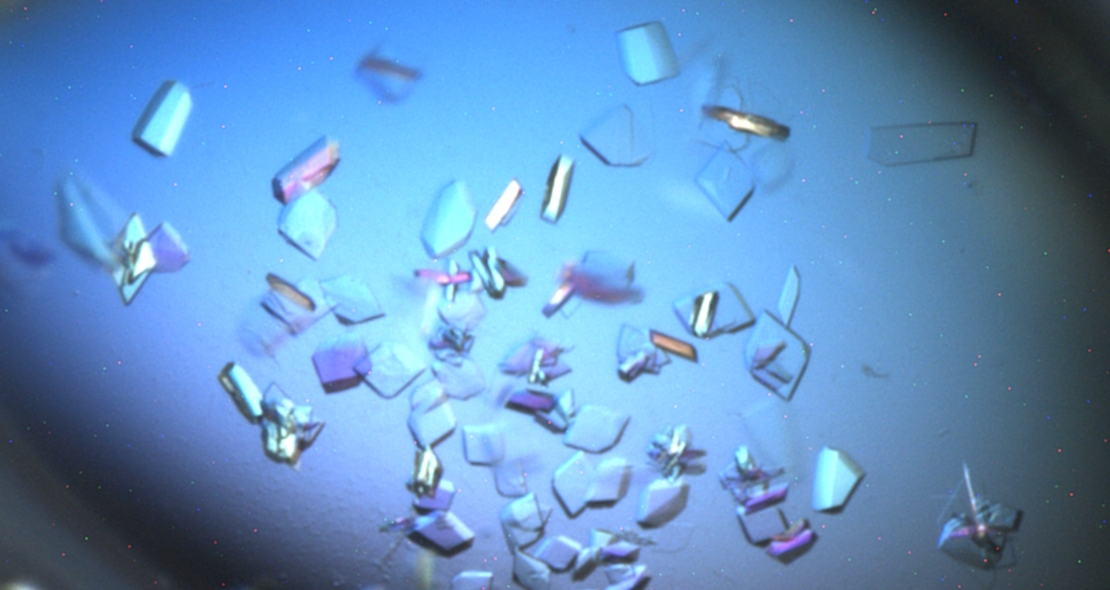
The trials, tribulations and joy of protein crystallisation
Despite the recent success of cryoEM in solving more protein structures, X-ray crystallography remains the best method to obtain high resolution (≤3 Angstroms) structures that can facilitate structure-based drug design. However, the method obviously requires the production of ordered, diffracting crystals and crystallisation has been flippantly eluded to as being more of an art as science.
 In order to obtain good diffraction, crystals that are in the order or 30-40 µM in three dimensions help enormously. Moving from a protein in solution to a suitable crystal can require the screening of many hundreds of different conditions, evaluating a number of key criteria generally in an iterative manner from initial “hits” or “leads”. Often these initial leads, which are unsuitable for data collection themselves (e.g. small needle-like, irregular, twinned, multiple crystals or even phase separation) can be improved on by iterative variations of the precipitant formulations. Seeding from poor crystals can produce drastic improvements in quality or even new crystal forms if used in further screening.
In order to obtain good diffraction, crystals that are in the order or 30-40 µM in three dimensions help enormously. Moving from a protein in solution to a suitable crystal can require the screening of many hundreds of different conditions, evaluating a number of key criteria generally in an iterative manner from initial “hits” or “leads”. Often these initial leads, which are unsuitable for data collection themselves (e.g. small needle-like, irregular, twinned, multiple crystals or even phase separation) can be improved on by iterative variations of the precipitant formulations. Seeding from poor crystals can produce drastic improvements in quality or even new crystal forms if used in further screening.
The key parameters that need to be evaluated are:
Protein factors; purity, concentration, stability and buffer all affect the outcome. In addition, it is frequently important to evaluate a number of different constructs and to set up the crystallisation trials with differently known inhibitors as both of these can and do affect crystallisation.
Precipitant i.e. the chemical cocktail in a screen which gives a crystal. Frequently these contain PEGs or salts. Easy options are to vary polymer chain length and/or the nature of building block, anions or cations in the salts; pH is also an important variable. Additive screens can be used as well as substitutions or relevant co-factors can be added to the drop.
Experimental set-up. Different formats can be explored e.g. sitting drops, hanging drops, in gels, lipidic cubic phases, robotically on 100-200 nl scale, larger scale with uL and pipettes. The ratios of protein:precipitant can have a big effect on crystal size and quality and temperature can be an important variable; we generally look at 4°C and 20°C.
Crystallisation is far from predictable, there are often surprises; unlikely conditions that work, thoroughly logical approaches that struggle to produce the desired effects.
And beauty is not always in the eye of the beholder, many ugly crystals can diffract well in the X-ray beam and many beautiful, visually perfectly, symmetrical crystals produce nothing. For a good introductory video on protein crystallography, have a look at the Royal Institution’s video.
Having evaluated thousands of conditions, following up on hits with secondary, tertiary and more screens, it is of course very satisfying to see diffraction to <3 Angstroms and then ultimately the beautiful sight of the structure, particularly if it is a novel structure or reveals novel information on how compounds are binding to the protein. At that point, the joy certainly exceeds the sum of the trials and tribulations along the way.
To find out more about how we tackle protein crystallisation, have a look at our services page.