Method Optimisation and Protein Production for a High Throughput Screen
A client requested assistance providing protein to enable a high throughput screen (HTS). This was for an emerging target that was implicated in the development of neurodegenerative diseases, strokes, and epileptic seizures. Their yields of purified protein from E.coli were around 1mg/L, with initial data suggesting that 250mg of purified protein would be needed to complete the HTS. We were asked to help optimise the protein expression and purification processes, as it was neither feasible or cost effective to supply and process these amounts of cell paste in the timescale required. Initial material was requested for assay development, after which a large-scale batch of homogeneous protein would be required.
Our Solution
Although the protein was expressed, yields were low and a large part of the protein was expressed in the insoluble fraction in E.coli. Also, purification was difficult, in part, due to low affinity for the purification resin. As part of the feasibility study, we were able to improve solubility and expression levels using different E.coli growth media and expression conditions. Optimisation of cell lysis also allowed us to recover a larger percentage of the soluble protein expressed. We also compared and selected different metal affinity media, and these alterations to the initial protocol improved the yield to ~8.5mg/L.
Even though this improvement was substantial and reduced the amount of total protein required, we still needed to process ~20L of cell paste in a timely manner to produce a single, homogeneous batch of protein. Processing at this scale can increase timelines and affect the quantity and quality of the protein produced, due to increased aggregation and degradation.
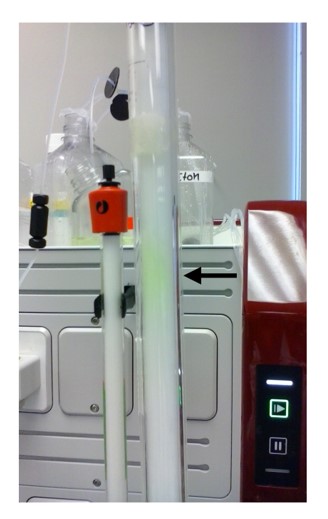
Fig 1. The active protein can be seen bound to its ligand as it passes through the SEC column
The Impact
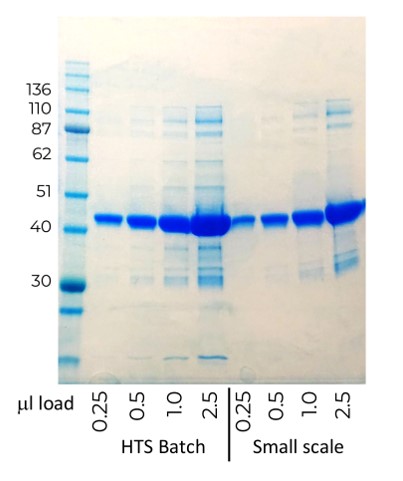
The protein provided from our feasibility study was found to be more active than the protein produced before optimisation, allowing successful assay development and reducing the amount of protein required for the HTS to 150mg. By using our optimised methods, and combining resources to allow parallel processing, we were able to successfully purify 20L of cell pellet to provide the customer with a single batch of over 200mg of protein to enable their high throughput screen on schedule. Purity and activity of the HTS protein batch was equivalent to that produced at small scale (Fig 1 below).
Fig 2. Comparison of protein purified from small scale and HTS scale expression grows