In vivo biotinylation of a challenging target in insect cells
Our client needed a biotinylated version of a highly disordered protein for their high-throughput screening assay. Previous work with the protein of interest had demonstrated issues with protein stability and loss of protein during concentration using centrifugal concentrators; we were therefore concerned about further losses through the increased processing required to biotinylate the protein in vitro. As we knew the protein expressed well in insect cells, we made the decision to try co-expressing the protein with BirA, to biotinylate it in vivo.
Expression and purification of the protein went well and we carried out intact mass analysis to check the identity and biotinylation of the purified material. Unfortunately however, this data was not easy to interpret due to the presence of several other post-translational modifications; we couldn’t tell if the increased mass was due to biotinylation or not!

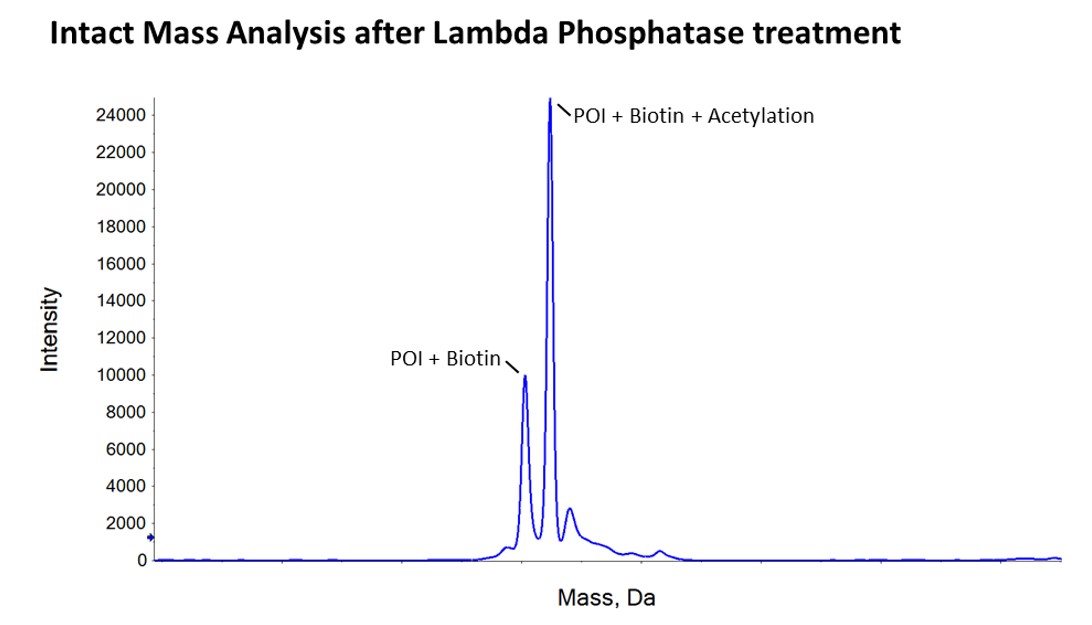
We then carried out peptide mapping on our in-house mass spectrometer, which told us that at least some of the protein was biotinylated but it was hard to be certain about how much of the material was biotinylated by this method. Fortunately, the client had also requested us to carry out an analytical lambda phosphatase treatment to assess whether the protein was phosphorylated. This removed 3-7 phosphates from each purified molecule, cleanly taking the protein back to two masses; one exactly as you would expect for the biotinylated protein and the other for the biotinylated protein plus an acetylation. This was a really nice confirmation that all of the protein had been biotinylated in vivo!…and provided very clear data back to the client that the in vivo biotinylation approach had worked successfully.
For further background information on use of biotinylation, have a read of our latest blog “Biotinylation of Recombinant Proteins”.