The Importance of Protein Construct Design
Early on in any recombinant protein expression and purification project, there are many factors that we at Peak Proteins consider as part of the protein construct design process. It cannot be understated the importance of committing time and effort to this stage, as the choices made are often absolutely critical to the ultimate success or failure of the project.
This case study highlights one relatively simple example where the placement of the tag was the key to the successful expression of a target protein for our client.
Our Solution
For the project in question, our client ideally wanted the full lumenal region of the target protein. They had already tried 3 different length constructs with different (mostly C-terminal) tag options but to no avail. They had achieved some low expression with N- terminal tags, but the protein had unfortunately suffered from severe proteolysis. The structure of the C-terminal domain had been solved and when we looked in detail at the papers, we observed that the C-terminal isoleucine forms an important part of the binding site. Therefore, placing a tag there was likely to generate a non-functional construct and/or destabilise the protein. Furthermore, the protein is a type II membrane protein and prior to the lumenal region is a helical signal anchor with a short cytoplasmic region. With both of these pieces of information, we proposed to use only N-terminal tags. We made a full lumenal construct of the protein with 2 different tag options and transfected the plasmid into HEK293 cells for expression. These were harvested earlier than usual with the aim of reducing the level of any proteolysis. Unfortunately, the construct with the Fc tag still had low expression and had suffered from proteolysis. However, the 6His tag version gave very good expression levels indeed, purified well and had no evident proteolysis. (Figure 1).
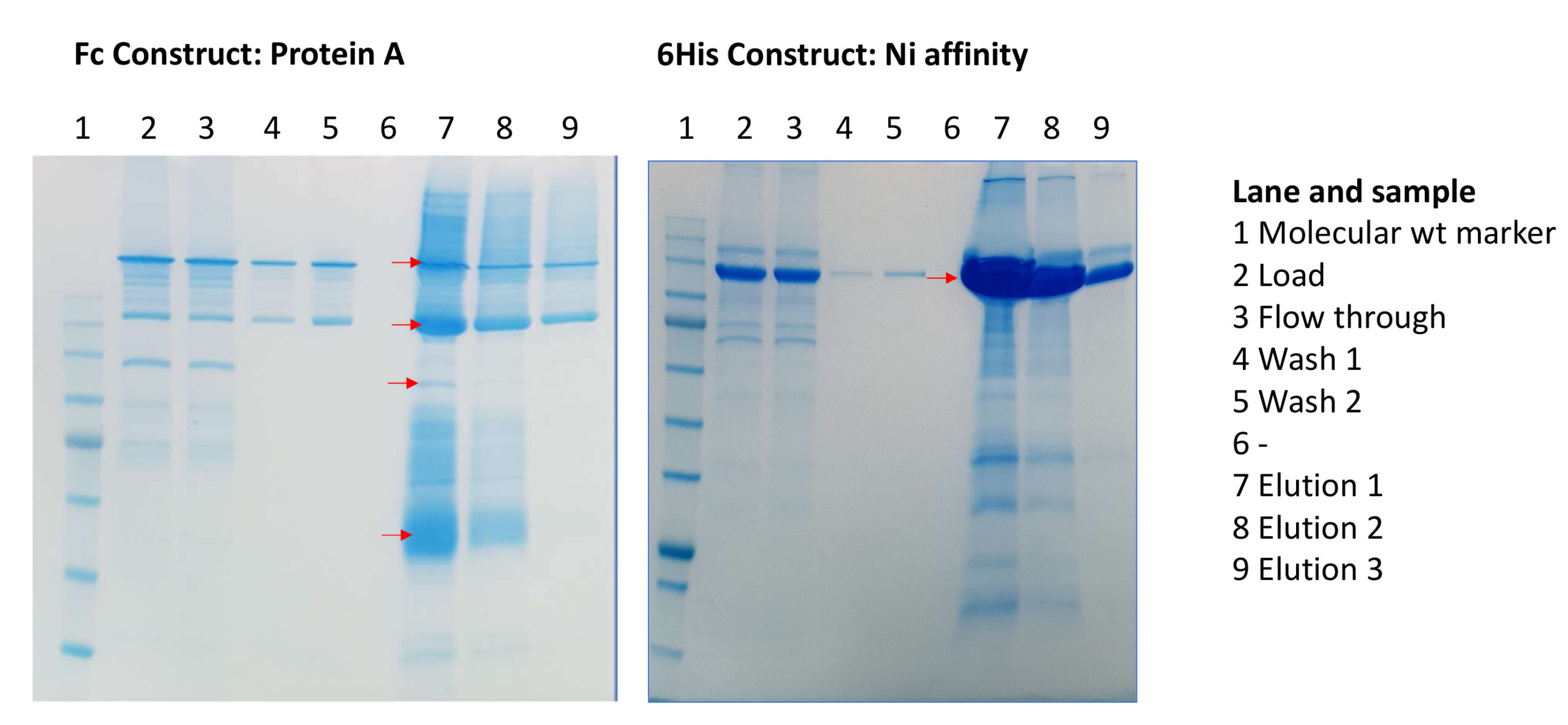
Figure 1: SDS PAGE of samples from initial chromatography step for Fc construct on a Protein A column and the 6His construct on a Ni metal affinity column. Red arrows indicate bands that were confirmed as the target protein by peptide mapping and mass spectrometry, confirming proteolysis had occurred with the Fc tagged construct.
The Impact
In the initial experiment the Ni metal affinity column had been overloaded due to the high expression levels obtained, with target protein observed in the flow through sample. This was accounted for in the scale up expression and purification run using a larger column. In this batch, the purified yield from the Ni metal affinity capture column was 88mg from 1L of a HEK293 culture (Figure 2). Subsequent polishing on a size exclusion column gave protein that was >95% pure and more than met the quality criteria specified by the client.
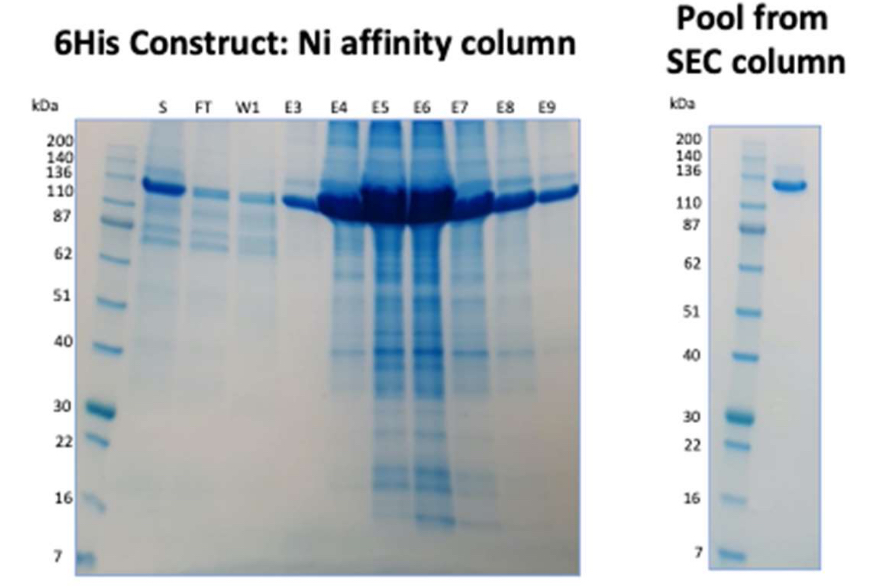
Figure 2: SDS PAGE from scaled up Ni metal affinity capture column of N term 6His tagged – target protein. (S: media supernatant from HEK cell grow, FT: flow through from column, W: Wash fractions, E: sequential elution fractions). Second gel shows the final purified material from a SEC column.


