Using Peptide Mapping to Reveal Glycosylated and Non-Glycosylated Protein Forms
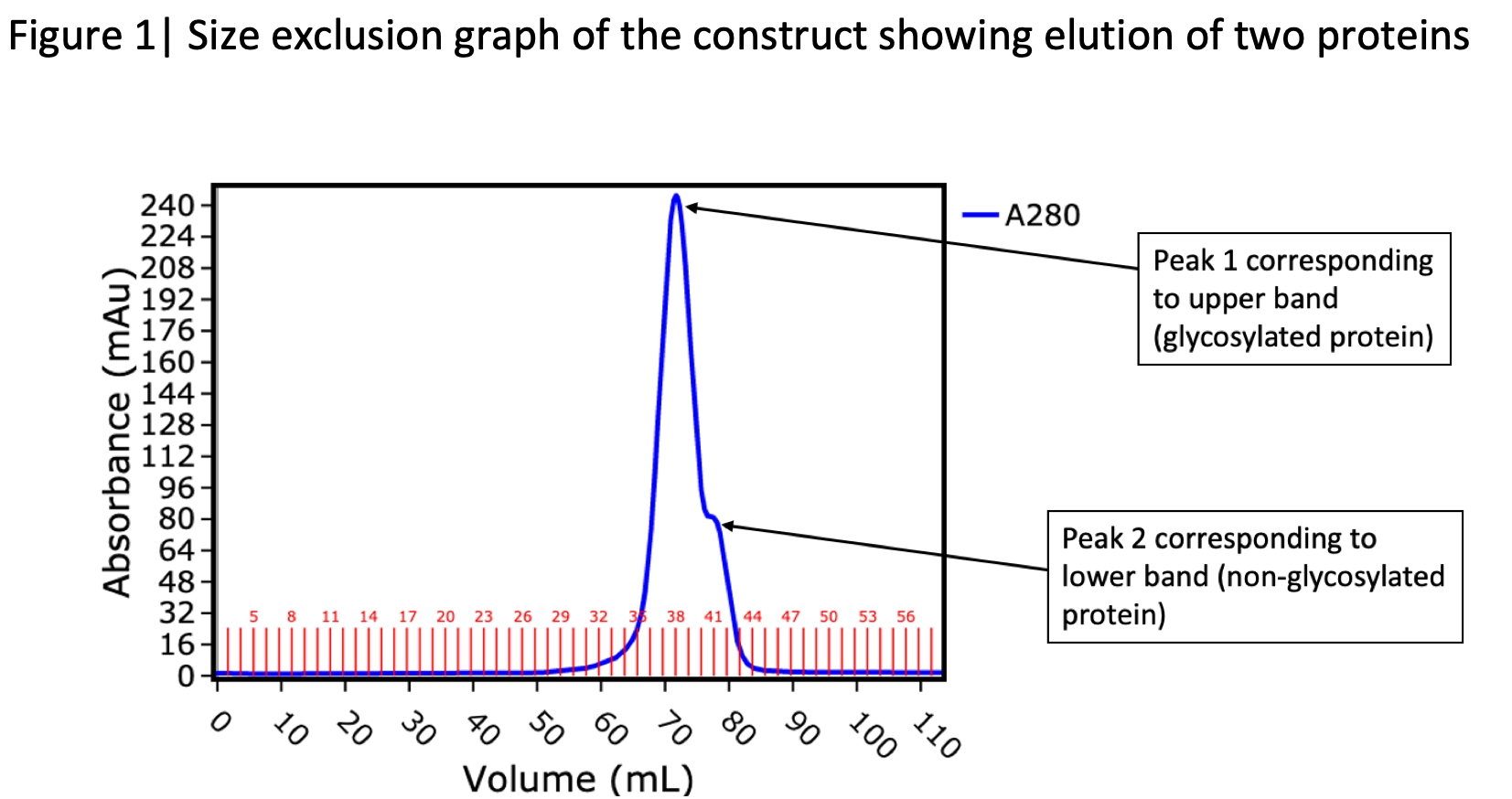
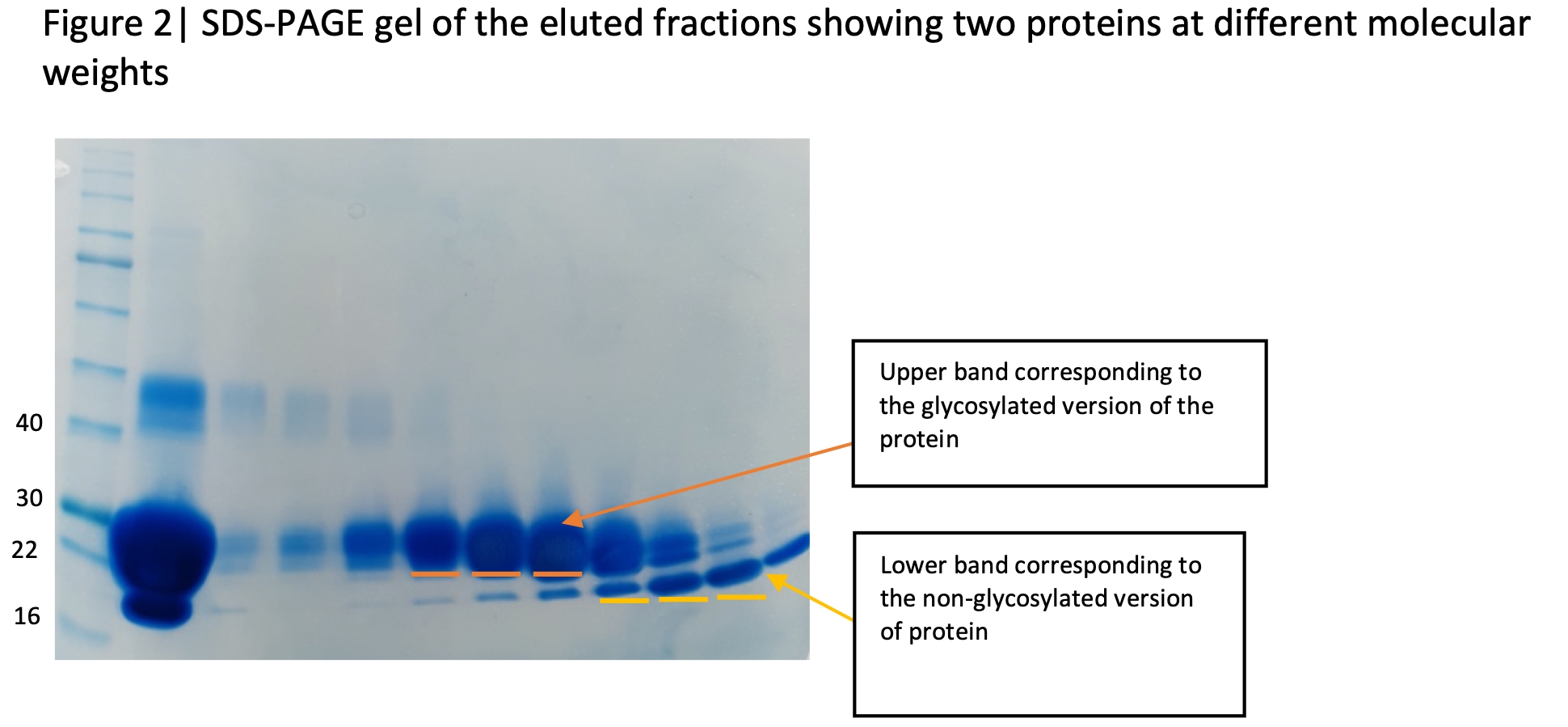
A client had requested us to express and purify a protein for assay studies. Six 6His tagged constructs were designed to increase the chances of obtaining a suitable form of protein that could be tested in assays. After E.coli expression and nickel sepharose affinity purification, one construct was found to have been expressed exceptionally well and was purified further via size exclusion chromatography (Figure 1). SDS-PAGE of the eluted fractions revealed two major bands (Figure 2). The more prominent band exhibited characteristics of classic glycosylated protein and as a result we identified this as the desired protein. The second band which was clearly distinguishable from our target protein was initially viewed as a contaminant as it was running at a lower molecular weight. The identity of the lower band was required to inform the next step of purification.
Our Solution
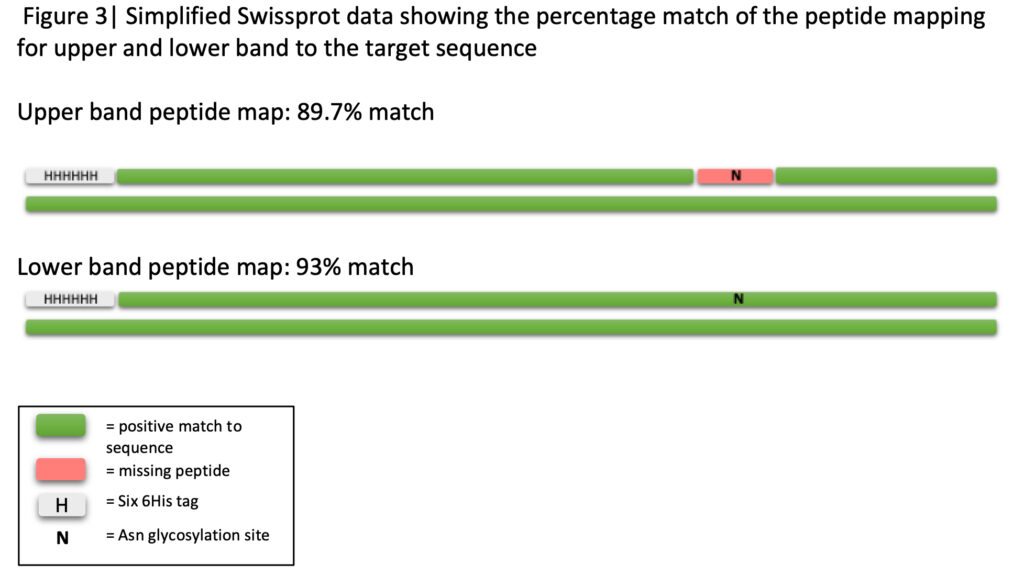
To identify this contaminant, the lower band of the SDS-PAGE gel was used for peptide mapping using a Sciex X500b mass spectrometer. The results surprisingly revealed that the identity of the contaminant was in fact our desired protein and data analysis from Swiss-Prot revealed a 93% match to the target sequence. A comparison of the peptide mapping of the upper and lower band from the gel revealed that the upper band had a specific peptide missing, indicating that this peptide may be glycosylated (Figure 3). Upon examining this region in the Uniprot database, the peptide contained an asparagine which is involved in N-glycosylation and therefore the protein was assumed to be glycosylated. The peptide mapping of the lower band had this specific peptide present which inferred that there was no glycosylation (Figure 3). As a result, we were able to identify both glycosylated and non-glycosylated versions of the protein, allowing both to be tested by the client.
The Impact
The use of peptide mapping of the protein, which was thought to have been a contaminant, was shown to be vital in identifying potentially valuable proteins for our clients. This shows the importance of investigating the identity of contaminants which are present in an SDS-PAGE gel as it can often be misleading. Once we knew that it was possible to get the non-glycosylated form of the protein, it then gave us two options to increase the yield of this form. One option for obtaining pure, non-glycosylated protein is to treat mammalian cells with Kifunensine which prepares oligosaccharides for cleavage by Endoglycosidase H. Alternatively, another option is to mutate the glycosylation sites within the amino acid sequence which prevents any possibility of any glycosylation events occurring which may interfere with purifying non-glycosylated protein. Both options are perfectly viable to create specific non-glycosylated proteins for clients.


