Why Everyone’s Talking About Metabolic GPCRs
Importance of metabolic GPCRs
Metabolic GPCRs play a vital role in how our bodies manage energy. These G protein-coupled receptors act like molecular switches, translating signals from hormones and nutrients into cellular actions that regulate everything from blood sugar to appetite. They’re involved in how we process food, store fat, and maintain energy balance. Due to not only their role in metabolism but also the recent development of blockbuster drugs, the metabolic GPCRs are some of the hottest targets in drug discovery. Moreover, cryo-EM has emerged as a revolutionary tool in structural biology, particularly for membrane proteins such as the metabolic GPCRs. These receptors – once considered structurally elusive due to their dynamic and membrane-bound nature – can now be visualised routinely at high resolution (<2.5Å) 1. Cryo-EM is particularly beneficial for the structural elucidation of agonist-bound active state GPCR complexes, transforming the way ligands are designed to target these receptors.
The headline-making weight-loss and diabetes drugs such as Ozempic, Wegovy and Mounjaro all target the Glucagon-like peptide-1 receptor (GLP-1R) which plays a crucial role in helping us to feel fuller for longer after eating. Cryo-EM structures were published in 2021 of Semaglutide, the active ingredient in Ozempic, bound to the GLP-1R receptor 2. The structural insights gained have now transformed the way medicines are designed putting structure-based drug design back at the forefront of drug discovery programs. Pharmaceutical programs are not just targeting the GLP-1R receptor. Researchers are now developing dual and triple agonists that also target the glucagon receptor (GCGR) and the glucose-dependent insulinotropic polypeptide receptor (GIPR), two key regulators of energy balance and glucose homeostasis. Example therapeutics include tirzepatide (approved as Mounjaro) and retatrutide (currently in clinical development) for the treatment of obesity and diabetes (Figure 1).
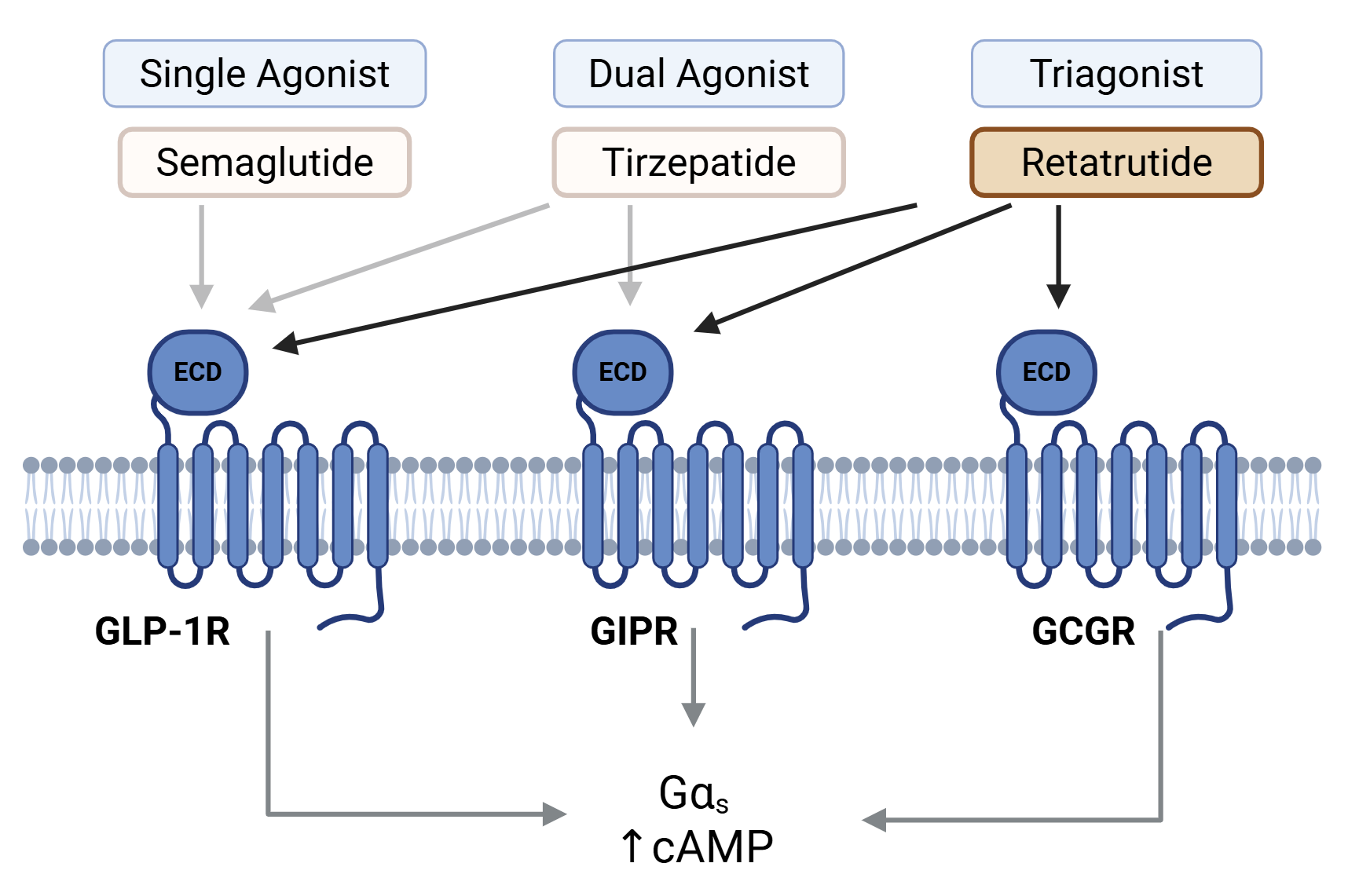
Figure 1: An overview of the single, dual and triple agonists that target the GLP-1R, GIPR and GCGR receptors.
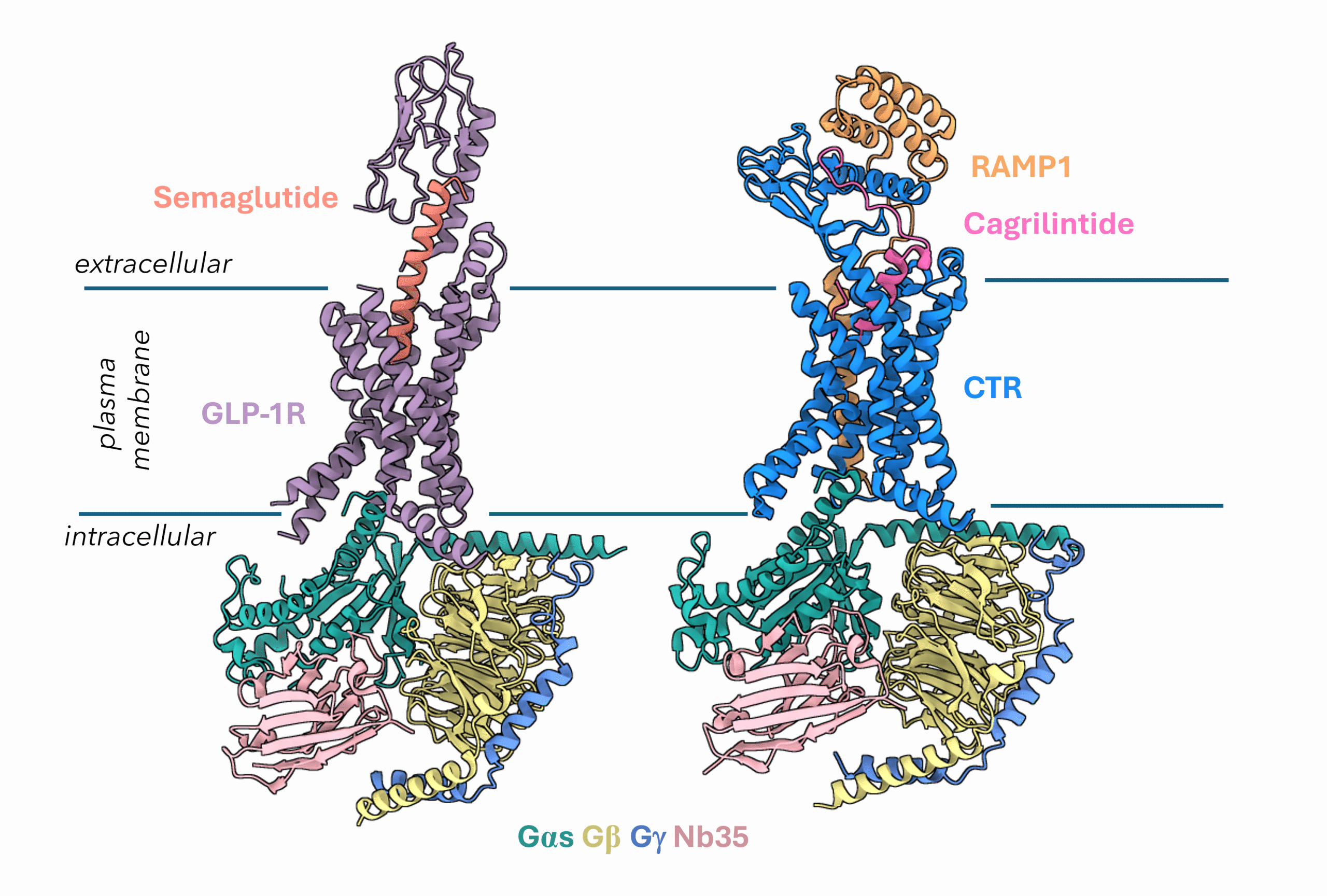
Another promising target for tackling metabolic diseases is the Amylin receptor. Amylin is a hormone co-secreted with insulin that helps reduce food intake, slow gastric emptying, and suppress glucagon secretion all of which are critical components of metabolic regulation. Pramlintide, used for the treatment of type 1 diabetes, is currently the only approved drug to target the amylin receptors. However, the amylin receptor has emerged as a powerful partner to GLP-1R. By targeting both receptors, researchers aim to achieve more sustained effects on weight loss, appetite control, and glucose regulation. One such combination therapy in development is Novo Nordisk’s CagriSema, which pairs cagrilintide (an amylin analogue) with semaglutide (Figure 2). This dual-agonist approach is designed to take advantage of the complementary actions of both hormones, offering a new frontier in the treatment of metabolic diseases.
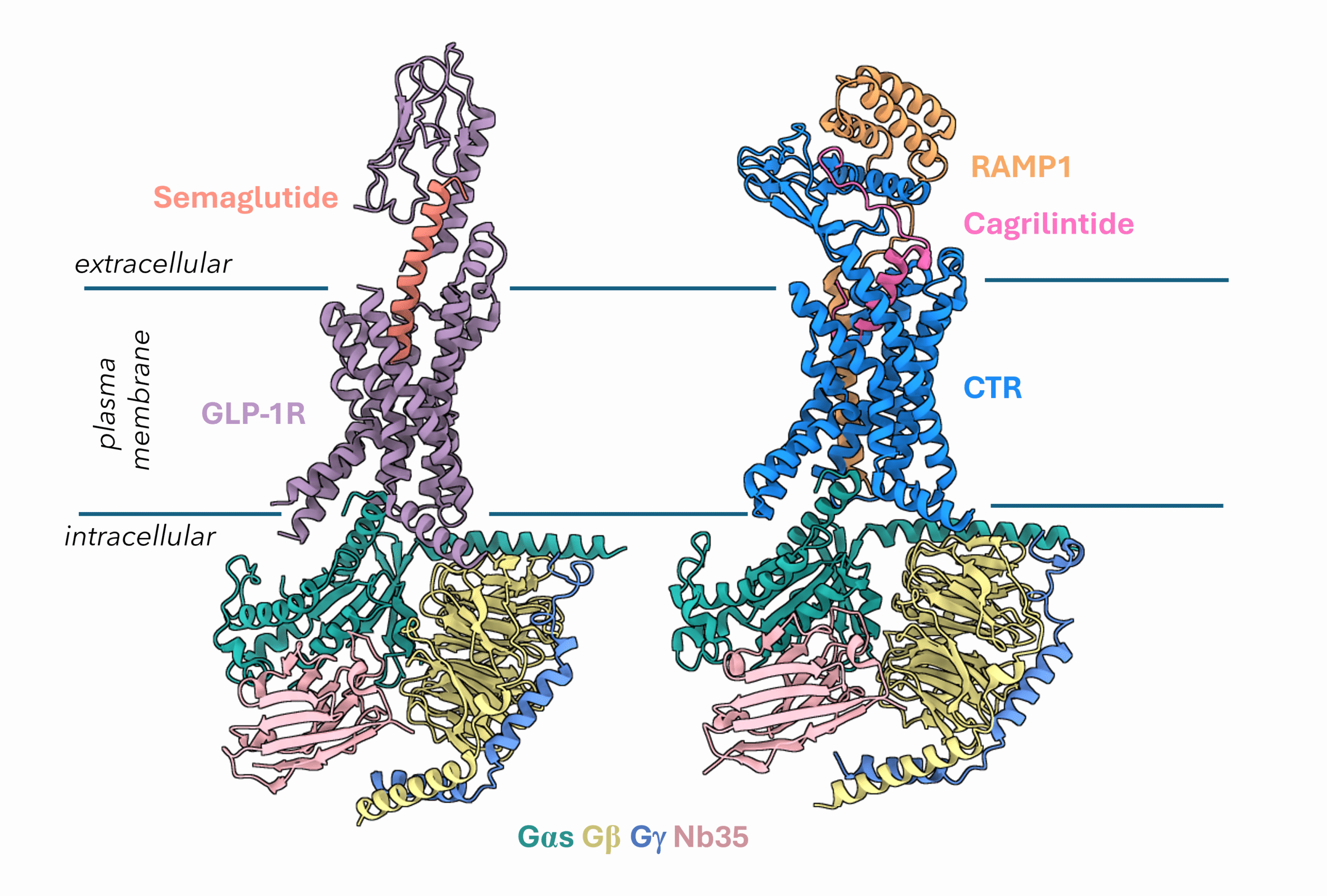
Figure 2: Cryo-EM structures of GLP1R bound to Semaglutide (PDB 7KI0) and AMY1R bound to Cagrilintide (PDB 9BP3).
Supporting your research
At Sygnature Discovery, our Protein Science department has extensive expertise in generating high-quality, cryo-EM ready samples of metabolic GPCRs. Our dedicated membrane protein team supports every stage of the workflow, from construct design and purification strategy to grid preparation and structure determination. To date, we’ve successfully produced over 200 samples across at least 20 different GPCR targets, supporting protein production for both high-resolution cryo-EM structural studies and for compound screening or biophysical studies. Among our team, Lead Scientist Dr. Rachel Johnson has published work featuring high resolution cryo-EM structures of key metabolic receptors, including GLP-1R3, 4 and the amylin receptor5, 6, which reflects the depth of expertise behind our approach. Our team is also experienced in generating apo receptor samples, which, through our integrated capabilities, can be applied to both cryo-EM structure determination and for biophysical screens such as SPR, ASMS or assay development.
Advance your research with Sygnature Discovery’s membrane protein expertise. From construct design to cryo-EM structure determination and biophysical screening, our in-house capabilities support every stage of your metabolic GPCR program. Contact us today on info@peakproteins.com to learn how our Protein Science services can strengthen your GPCR drug discovery efforts and accelerate structural insights from compound binding.
References:
- 1Danev, R., Belousoff, M., Liang, YL. et al. Routine sub-2.5 Å cryo-EM structure determination of GPCRs. Nat Commun 12, 4333 (2021). https://doi.org/10.1038/s41467-021-24650-3
- 2Zhang X, Belousoff MJ, Liang YL, Danev R, Sexton PM, Wootten D. Structure and dynamics of semaglutide- and taspoglutide-bound GLP-1R-Gs complexes. Cell Rep. 2021 Jul 13;36(2):109374. doi: 10.1016/j.celrep.2021.109374. PMID: 34260945.
- 3Zhang X, Johnson RM, Drulyte I, Yu L, Kotecha A, Danev R, Wootten D, Sexton PM, Belousoff MJ. Evolving cryo-EM structural approaches for GPCR drug discovery. Structure. 2021 Sep 2;29(9):963-974.e6. doi: 10.1016/j.str.2021.04.008. Epub 2021 May 5. PMID: 33957078.
- 4Johnson RM, Zhang X, Piper SJ, Nettleton TJ, Vandekolk TH, Langmead CJ, Danev R, Sexton PM, Wootten D. Cryo-EM structure of the dual incretin receptor agonist, peptide-19, in complex with the glucagon-like peptide-1 receptor. Biochem Biophys Res Commun. 2021 Nov 12;578:84-90. doi: 10.1016/j.bbrc.2021.09.016. Epub 2021 Sep 16. PMID: 34547628.
- 5Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. doi: 10.1126/science.abm9609. Epub 2022 Mar 25. PMID: 35324283.
- 6Cao, J., Belousoff, M.J., Johnson, R.M. et al. Structural and dynamic features of cagrilintide binding to calcitonin and amylin receptors. Nat Commun 16, 3389 (2025). https://doi.org/10.1038/s41467-025-58680-y
- 7Cao J, Belousoff MJ, Gerrard E, Danev R, Fletcher MM, Dal Maso E, Schreuder H, Lorenz K, Evers A, Tiwari G, Besenius M, Li Z, Johnson RM, Wootten D, Sexton PM. Structural insight into selectivity of amylin and calcitonin receptor agonists. Nat Chem Biol. 2024 Feb;20(2):162-169. doi: 10.1038/s41589-023-01393-4. Epub 2023 Aug 3. PMID: 37537379.