Developing a robust biopharmaceutical production process
In this case study we describe the journey to develop a process for the production of the protein component of a biopharmaceutical that was suitable for full scale manufacture. The initial laboratory scale process we were presented with was not fit for purpose and required some deep understanding of the target protein, ultimately leading us to switch the host cell expression system and re-engineer the construct, and we are quite proud of the result.
Background to the study
Here at Sygnature Discovery we have extensive experience in producing recombinant proteins for the many and varied end uses required by our customers. Our client, in this instance, reached out to us with a request to develop a process that was suitable for full scale manufacture of the protein component of a biopharmaceutical. The protein in question is a small, secreted <10kDa glycosylated protein, that contains disulphide bonds and a region near the N- terminus that is essential for activity. Ultimately it is to be site specifically conjugated to a payload to generate the final API (active pharmaceutical ingredient).
Biologics represent a rapidly evolving field in medicine, exemplified by the fact that in 2023, 30% of all drugs approved by the FDA were biologics. They include a wide range of products such as vaccines, blood components, gene therapies, tissues, and recombinant proteins [1]. Recent advancements in biologics have led to the development of innovative treatments like monoclonal antibodies and antibody derived molecules which can precisely target cancer cells, and CAR-T cell therapies, which modify a patient’s own immune cells to fight cancer more effectively [2].
We follow “Quality by Design” [3] principles when working on biopharmaceutical projects, with the firm belief that the more robust and simple you can make a process, the more likely you are to generate a manufacturing process that is easier to control, less prone to expensive deviations, and is capable of producing batch after batch of high quality active product.
The Challenge
With that in mind, our aims for this project included the following desirable features, all aimed to generate early in the development of this API an excellent SISPQ (Safety, Identity, Strength, Purity, and Quality) profile along with a process that was reproducible, scalable, and relatively cheap to produce at an industrial scale.
Initial work
Based on the literature along with the laboratory work previously performed by our customer, our preliminary investigations focussed on expression of the protein in E. coli via either soluble expression or expression targeting inclusion bodies. We even explored expression to the periplasm by inclusion of relevant signal peptides [4 – 7] on some constructs. E. coli is relatively inexpensive and is frequently used at an industrial scale for the production of high value compounds in biotechnology [8, 9]. Despite successful production of fully active protein, with disulphide bonds formed in E. coli, both by refolding from inclusion bodies and via a soluble expression the yields were low ~1-2 mg per L culture, multiple species were observed by intact MS and multiple purification steps were required to purify the protein. Taken together, the data collected suggested that expression of the protein in E. coli would not be practical at an industrial scale and the benefits of inexpensive expression would be quickly consumed by the need for increased culture volumes and extensive purification schematics. In addition E. coli would present a greater challenge to meet endotoxin level targets at scale. We had successfully transferred the protocols used by our client to our labs, and had expanded the options for production, however none of them met our criteria for success and so were not fit for purpose.
Changing the expression host
Our investigation then moved towards expression of the protein in mammalian cells, namely the HEK293 and CHO cell lines we routinely use. We assessed three different signal sequences and concluded that use of the Native signal sequence in HEK293 was the most optimal system to use achieving a yield of ~100 mg per L culture (Figure 1a).
Secreted expression of the protein in HEK293 cells also aided the cost of purification, with a single ion-exchange chromatography column required to both capture and purify the protein from other supernatant components (Figure 1b). Furthermore, the key N-terminal region of the protein was in the correct form and testing by our client confirmed we were producing very active product.
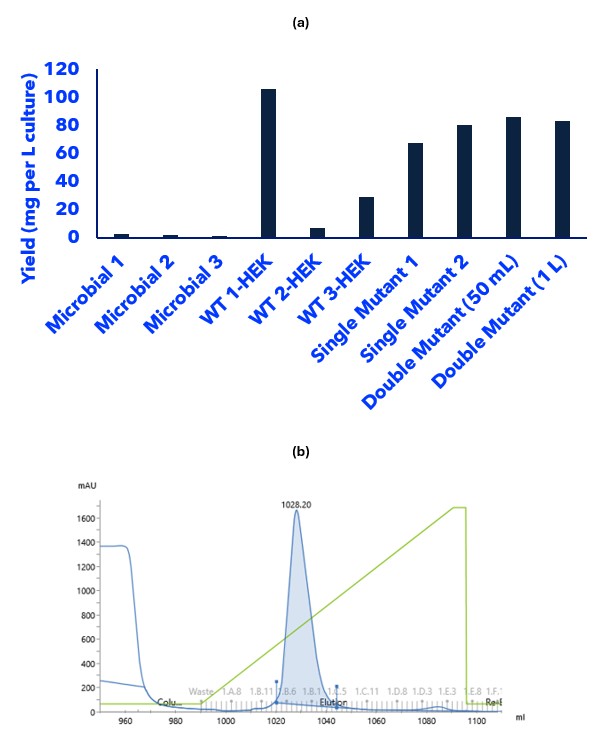
Figure 1. The desired expression and purification system was required to be high yielding and relatively inexpensive to produce. (a) Pivoting from microbial (E. coli) expression of the protein to mammalian expression significantly increased the yield of protein produced. (b) Secreted mammalian expression enabled a single ion-exchange chromatography column to be used to both capture and purify the protein to a high yield and purity.
Further engineering the construct
However, multiple O-glycosylated post translational modifications were observed by intact MS (Figure 2a). In order to help with a smoother regulatory approval, we recommended to the client that these were removed, aiming to produce a consistent single species as observed by MS. Based on our previous experience with a similar protein we suspected that certain Thr residues were being glycosylated. We therefore systematically mutated these Thr residues to Ala (Figure 2b-c) and were rewarded with success. A double mutant variant of the protein removed all O-glycan modification and produced a single species (Figure 2d).
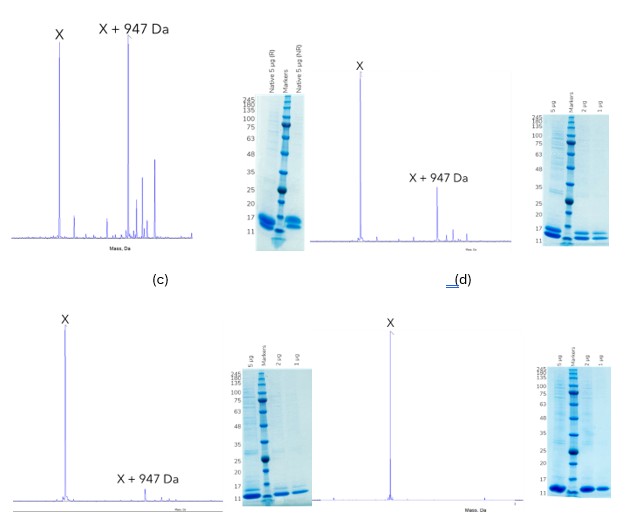
Figure 2. Expression of the protein in HEK293 cells produced multiple post translationally modified species, which were observed by intact MS and SDS-PAGE. Mutation of select Thr residues near the C-terminus to Ala residues removed the O-glycan modifications. (a) WT protein, 50 % unmodified, 50 % modified (b) Single mutant 1, 70 % unmodified, 30 % modified (c) Single mutant 2, 95 % unmodified, 5 % modified (d) Double mutant, 100 % unmodified, 0 % modified, highly pure product.
Confirmation run
Having established a high yielding and reproducible expression system for the protein; we assessed the scalability and low endotoxin potential for the system. Moving from 50 mL to 1 L cell cultures proved the system was scalable with ~80-100 mg per L culture yields being achieved. An endotoxin quantification assay showed that low endotoxin conditions had been achieved 0.1 EU/mL.
The impact
The work outlined above in no way fully describes the huge amount of effort and intellectual input that were key to delivering this project. The result; we in collaboration with our client, have successfully developed a scalable expression and purification system for the production of a protein for subsequent conjugation into a biologic API. The protein product produced features all of the desired traits for excellent SISPQ: fully active, amenable to subsequent conjugation, high yielding (~80-100 mg per L culture), low endotoxin (0.1 EU/mL), a single species observed by MS, reproducible, scalable (50 mL to 1 L cultures assessed), and relatively inexpensive for CoG (single step purification).
If you have a protein based biopharmaceutical project in early development that would benefit from our input then please don’t hesitate to get in touch info@peakproteins.com
References
[1] What Biologic Therapy Is and How It Works https://www.verywellhealth.com/biologics-or-biological-agents-2615117
[2] What are biologic drugs and how do they work? https://www.drugs.com/medical-answers/what-biologic-drug-3565613/
[3] A. S. Rathore and H. Winkle, “Quality by design for biopharmaceuticals,” Nat Biotechnol, vol. 27, no. 1, pp. 26–34, Jan. 2009, doi: 10.1038/nbt0109-26.
[4] A. Karyolaimos et al., “Enhancing Recombinant Protein Yields in the E. coli Periplasm by Combining Signal Peptide and Production Rate Screening,” Front Microbiol, vol. 10, Jul. 2019, doi: 10.3389/fmicb.2019.01511.
[5] M. Jeiranikhameneh, F. Moshiri, S. K. Falasafi, and A. Zomorodipour, “Designing signal peptides for efficient periplasmic expression of human growth hormone in Escherichia coli,” J Microbiol Biotechnol, vol. 27, no. 11, pp. 1999–2009, Nov. 2017, doi: 10.4014/jmb.1703.03080.
[6] K. Mirzadeh et al., “Increased production of periplasmic proteins in Escherichia coli by directed evolution of the translation initiation region,” Microb Cell Fact, vol. 19, no. 1, Apr. 2020, doi: 10.1186/s12934-020-01339-8.
[7] W. Zhang, J. Lu, S. Zhang, L. Liu, X. Pang, and J. Lv, “Development an effective system to expression recombinant protein in E. coli via comparison and optimization of signal peptides: Expression of Pseudomonas fluorescens BJ-10 thermostable lipase as case study,” Microb Cell Fact, vol. 17, no. 1, Mar. 2018, doi: 10.1186/s12934-018-0894-y.
[8] Bell, E. L et al. 2021. Biocatalysis. Nature Reviews: Methods Primer. 1. 46.
[9] Devine, P.N. et al. 2018. Extending the application of biocatalysis to meet the challenges of drug development. Nature Reviews: Chemistry. 2. 409-421


