Cautionary tales of secreted proteins in baculovirus infected insect cells
The baculoviral expression vector system (BEVS) was first introduced in the 1980s. It is widely used to generate many types of protein including intracellular, secreted and membrane proteins particularly GPCRs. As BEVS is a eukaryotic system it is capable of many post translational modifications, although glycosylation is very different to that in mammalian complex structures. A recent review has summarised the system and genetic engineering approaches used to improve it.
When working with secreted proteins we routinely use the HEK293-6E and CHO-3E7 systems. But we do sometimes have a need to secrete a protein from insect cells; the glycosylation may be preferable for crystallisation, expression may be known to be higher or a client might specifically request it. We have recently had a couple of examples of secreted proteins with extended tags, which gave data that was initially difficult to interpret but was most likely a consequence of some or all of the tag being degraded proteolytically.
Example 1
In the first example we secreted a protein with a C-terminal 3C protease-10His-TwinStrep tag. The protein was expressed in Hi5 cells and the purification attempted using either streptactin or Ni affinity chromatography. The results were very irreproducible. An initial small-scale feasibility suggested that expression levels were reasonable, however when this was scaled up to 3L no purified protein was observed after streptactin affinity chromatography.
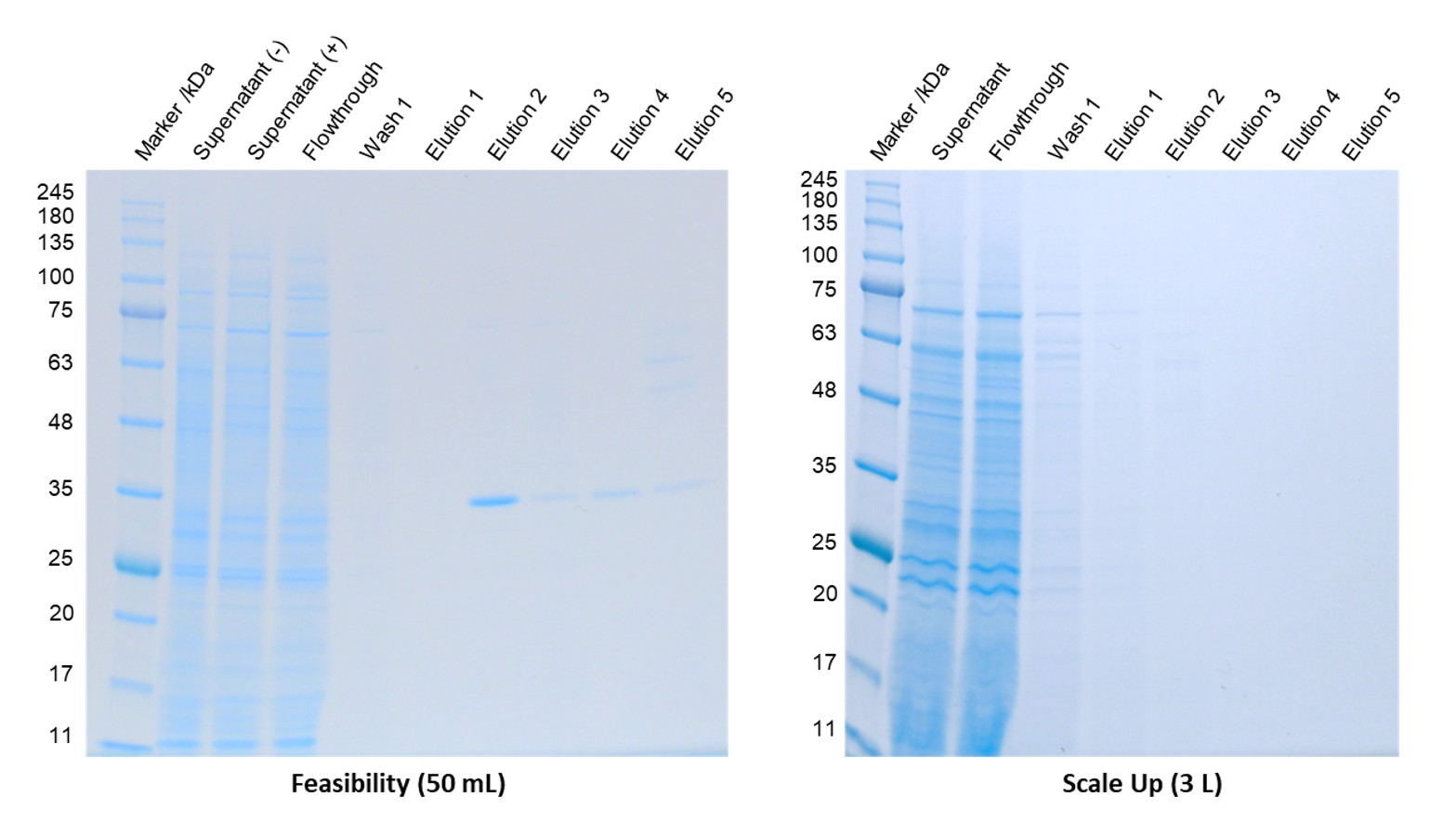
Figure 1: Comparable PAGE showing in process samples from small scale feasibility (left) and scale up (right)
When we analysed the intact mass of the feasibility sample the main species had the exact mass we expected. However, we also saw a species with a mass 4838 Da smaller, which equates to a protein that has been proteolytically cleaved after the phenylalanine in the 3C cleavage sequence (rather than at the glutamine-glycine 3C cleavage site).
We then performed an expression time course study where we harvested the protein from the same flask at different time points. Protein could be detected in the streptactin elution samples when cells were harvested at 48h but not at 72h or 96h, indicating proteolysis was occurring.
Example 2
In the second example we produced 2 versions of a protein; one had a C-terminal 10His-Avi tag designed for in vitro biotinylation (for immobilisation on an SPR chip) and the other had a 6His tag for X-ray crystallography. The proteins were expressed in Sf9 cells and purified by a combination of Ni affinity, ion exchange and size exclusion chromatography.
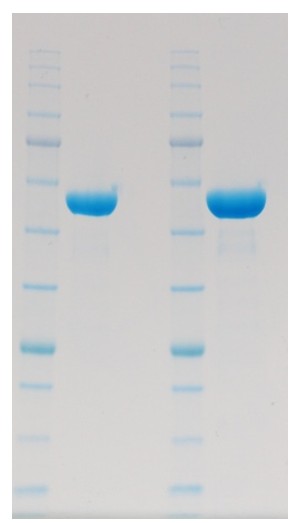
Figure 2: PAGE showing final products produced. (Left) C-terminal 10His-Avi construct (Right) 6His tagged construct
Both proteins expressed and purified very similarly using the His tag as the first step, with masses on SDS-PAGE and thermal melt behaviour as expected. However, the Avi-tagged protein was almost impossible to immobilise on a streptavidin surface. Despite the mass spectrometry being difficult to interpret (due to 4 N-glycan sites of exact structural identity unknown) the difference in intact masses of the proteins was clear. The crystallography construct with a 6His tag had a mass 415 Da less than the SPR construct with a 10His-Avi tag. The difference should theoretically have been 2610 Da, which suggested all of the Avi tag had been lost. This protein was harvested 72h after infection at which time viability was 70% or less.
Summary (A warning?)
Clearly any host cell will have the potential to cause proteolysis of target proteins. We think there are two contributory factors that have resulted in the problems we have observed.
1. Long, extended and by nature unstructured tags are much more vulnerable to proteolysis.
2. The culture medium of baculovirus infected insect cells is particularly rich in proteases, especially with prolonged harvest points beyond 48h. This can be because v-cath cathepsin (from the baculoviral genomes) accumulates in the secretory pathway and is secreted along with the recombinant protein of interest. However, even in baculoviral genomes where v-cath has been deleted (like flashBAC ULTRA), viral infection of insect cells is a lytic process especially in the later stages of infection. Hi5 cells in particular are known to release more than three times the amount of proteases as Sf9 and Sf21 cells.
In summary then, extreme caution should be taken when secreting recombinant proteins with long unstructured tags from baculovirus infected insect cells. Our team of experts can help with designing alternative tags, and exploring other expression systems when such problems arise.


