An Alternative Method To Confirm Protein Biotinylation
Biotinylation of Proteins
Biotinylation of proteins is the process of covalently binding a biotin molecule to a protein. At Peak Proteins we can biotinylate purified proteins enzymatically using recombinant BirA or alternatively biotinylate them intracellularly even when secreted.
The completion of biotinylation is usually assessed by mass spectrometry (intact mass analysis and if required peptide mapping). However, it is not always possible to carry out the assessment by intact mass analysis (e.g., when glycosylation is present or due to the size of the protein). Similarly, there may be reasons that peptide mapping data might not always give a clear answer or that we can’t actually perform the peptide mapping e.g., limited available protease sites in the target sequence of the protein of interest (POI).
We have therefore looked to develop a simple orthogonal test to confirm that we have successfully biotinylated a target POI and this method is described below.
When intact mass analysis is inconclusive
In this case study, an extracellular protein was purified by nickel and size exclusion chromatography and biotinylated intracellularly. The protein has multiple glycosylation sites which made it impossible to detect the mass with intact mass analysis using our Sciex X500B mass spectrometer.
Intact Mass Analysis

Figure 1: Intact Mass Analysis Results
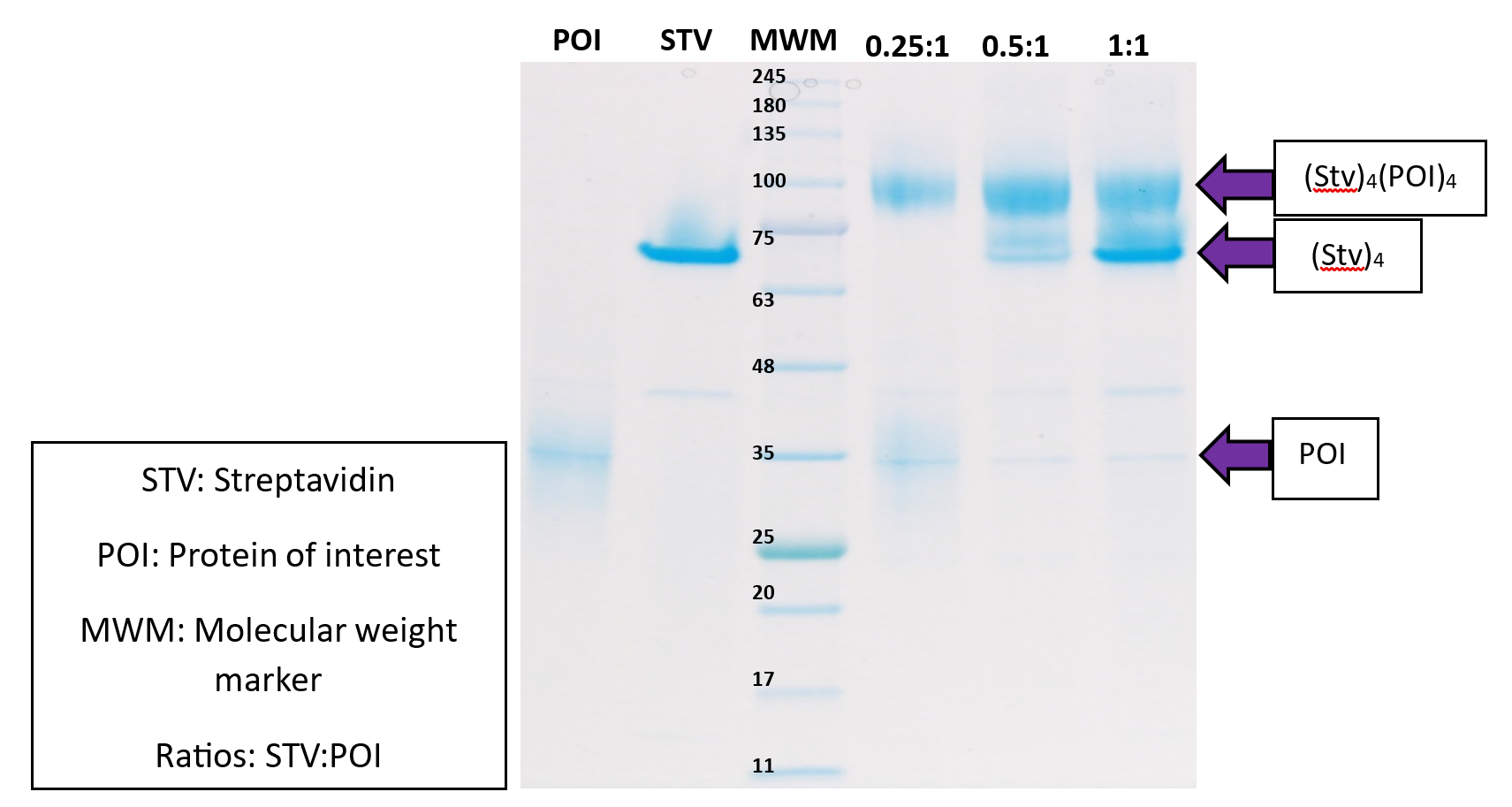
Biotinylation detection by SDS-PAGE
We decided to try an alternative method to detect the biotinylation of the target protein. The assay works by incubating the biotinylated protein of interest with Streptavidin, a tetrameric protein which can bind 4x the biotinylated POI and the resultant increase in mass of the protein complex can be detected by SDS-PAGE. This simple gel shift assay now allows us to confirm biotinylation of the POI as well as to quantify the degree of biotinylation.
Conclusion
This assay has proven to be useful as another tool to confirm the biotinylation of intracellular biotinylated proteins, and to determine whether an additional in vitro biotinylation step is necessary. This is especially useful when mass spectrometry methods are unsuccessful in monitoring the level of biotinylation for any reason.
If you are interested in expressing biotinylated proteins, please get in touch with us at info@peakproteins.com.


