Optimising Endoglycosidase H Treatment of Proteins – All Thanks to Cauliflowers!
In a previous case study, we have described the benefits of using the HEK293 suspension cell system in combination with the mannosidase inhibitor, kifunensine, and endoglycosidase H (EndoH) treatment to successfully crystallise complex proteins by removing bulky, N-linked oligosaccharides. Increasingly, we are relying on this approach to facilitate crystallography with more projects requiring secreted, correctly folded proteins. Although successful in the majority of cases, we recently hit a brick wall with this exact method and needed to develop a new route to tackle this problem.
Our Solution
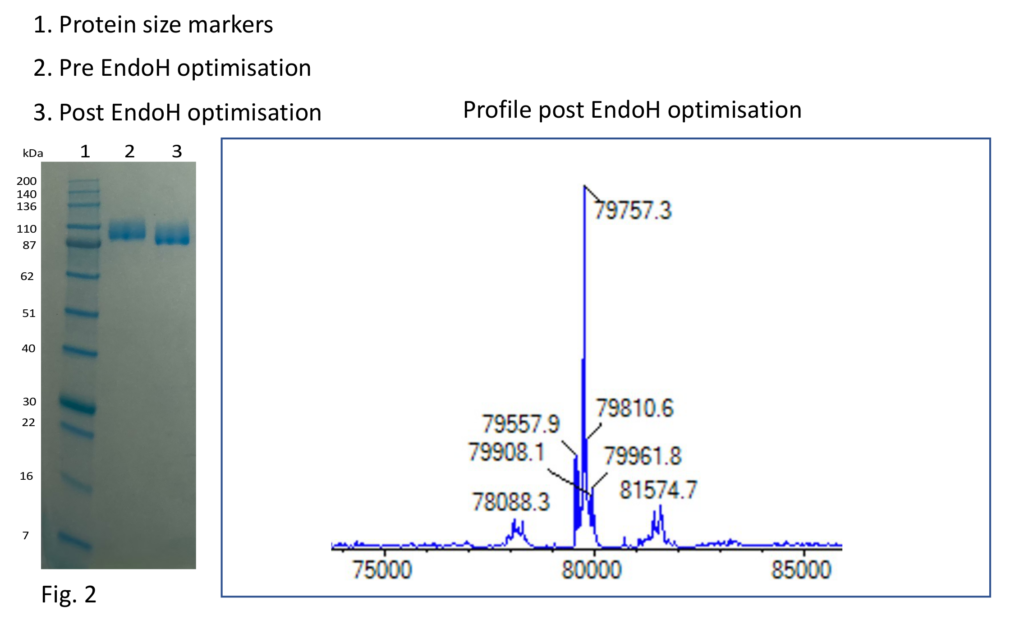
The purification was simple enough, containing two steps: nickel affinity and size exclusion chromatography. For the protein to be deglycosylated, the protein was treated with EndoH following elution from the nickel column. To determine the success of the treatment, both SDS-PAGE and protein mass spectrometry were employed. Here, a reduction in protein size and hence a downward shift in the gel band can be seen, but when we looked in more detail at the mass spectrometry data, we could see that only a partial deglycosylation had occurred as evidenced by multiple peaks, even though our usual procedure had been performed, and resulting in the blurred band seen post EndoH treatment on the SDS-PAGE (Fig.1).
The Impact
Following an unsuccessful round of crystallography, we wondered if the heterogeneity of the protein sample could be preventing crystallisation. To approach this problem, we first had to determine how to optimise the EndoH reaction. The repeating mass difference of 1662 Da between the various protein species that had been produced, led us to the publication: N-linked glycosylation of native and recombinant cauliflower xyloglucan endotransglycosylase 16A (Henriksson et al., 2003).
From this publication, we learned the 1662 Da difference correlated to the glycan in the GlcNAc1Man9, which should be cleaved off during treatment with EndoH. Further in-house research confirmed that the mass of the glycan does come to 1662.45 Da, meaning that our sample needed an improved EndoH treatment.
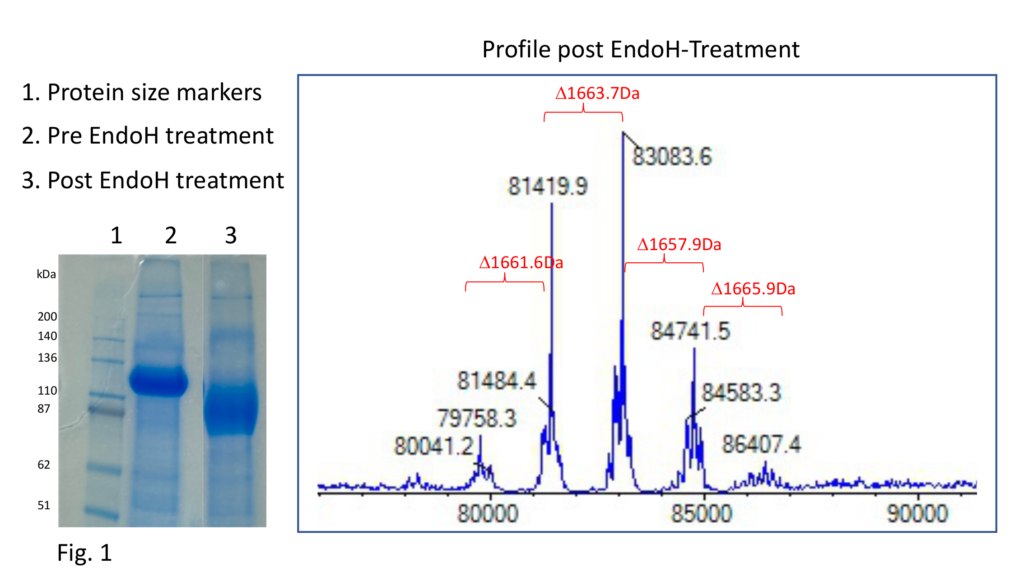
We tested three conditions to improve the activity of EndoH. The best results were seen using a shorter incubation time, at a higher temperature, and lower pH. Taking mass spectrometry readings over specific time periods, we could follow the transition of several mass species to a single mass peak (Fig. 2). A second piece of data suggesting the trial was a success was that a further downward gel band shift was also observed
Crystallography trials are currently on-going, but it is hoped that the improved homogeneity of the sample will facilitate successful crystal growth