Generating a TL1A Crystal Structure
At Sygnature Discovery, we thrive on solving complex protein challenges—and our recent success in solving the crystal structure of TL1A, a member of the TNF ligand superfamily, is a perfect example of how our expertise can accelerate drug discovery.
Background
TNF-like 1A (TL1A), also known as TNFSF15, is a cytokine that belongs to the tumour necrosis factor family of proteins, sharing homology with TNFα . It has a broad range of functions, being involved in maintaining cellular homeostasis, inflammatory responses and determining cellular fate (1). Its dysregulation has been implicated in multiple auto-immune diseases, atherosclerosis, rheumatoid arthritis and cancer, making TL1A an attractive therapeutic target (1-2).
The project began with a thorough review of the literature and structural databases to understand the behaviour of TL1A and any known challenges. In vivo, TL1A is expressed as a 251 amino acid transmembrane anchored protein that forms non-covalent homotrimers through its TNF homology domain and contains one intramolecular disulphide bond between cysteine residues, C95 and C135 (2-3). The N-terminal transmembrane domain (residues 1-71) is responsible for anchoring TL1A to the plasma membrane, whilst the C-terminal portion (residues 72-251) forms an extracellular soluble domain. The C-terminal portion can be cleaved off from the transmembrane domain by metalloproteinases such as TNFα converting enzyme (TACE), generating a soluble species of TL1A, known as sTL1A (residues 72-251) (3-4). sTL1A is the species that is commonly used in recombinant protein production, with wild-type sTL1A expressed previously (2-3). However , Zhan et al reported disulphide bond heterogeneity between C95 and C135, when wild-type sTL1A was expressed in E. coli. As such, they engineered a C95S/C135S mutant to prevent disulphide mispairing (5).
Guided by these literature precedents and prior experience with TNFα production, we designed two constructs: wild-type TL1A and a C95S/C135S mutant.
Purification and Quality Control
Both constructs expressed well, forming trimeric TL1A as expected. However, the wild-type protein showed increased aggregation and formed incorrect intermolecular disulphide bonds between TL1A protomers, as revealed by our non-reducing SDS-PAGE analysis (Figure 1). Whereas the SDS-PAGE and SEC-MALS analysis of the C95S/C135S mutant confirmed a homogeneous protein preparation, that retained the correct trimer formation (Figure 2). As such, the C95S/C135S construct was ideal to take forward for crystallography studies.
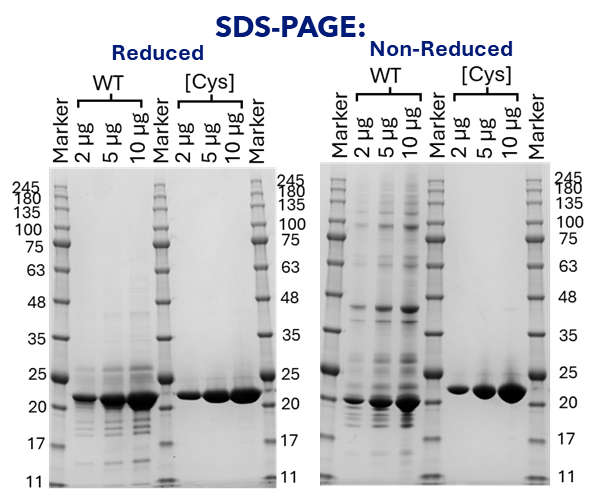
Figure 1: Reducing and non-reducing SDS-PAGE analysis of wild-type TL1A (WT) and the C95S/C135S double cysteine mutant [Cys].
WT TL1A and the C95S/C135S mutant were analysed under reducing (left panel) and non-reducing (right panel) conditions. Each panel includes molecular weight markers (Marker in kDa) and lanes containing 2 µg, 5 µg, and 10 µg of protein. Under reducing conditions, both WT and C95S/C135S migrate predominantly as a single band consistent with their expected monomeric molecular weights (~20.5 kDa). This reflects the disruption of non-covalent trimeric interactions due to the presence of reductant and SDS in the gel. However, under non-reducing conditions, purification artefacts are observed for WT, indicating the formation of incorrect intermolecular disulfide-linked species. Whilst a single band was present for the C95S/C135S mutant under both conditions, indicating its suitability for crystallography trials.
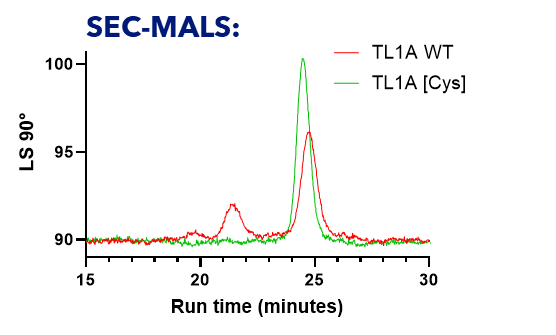
Figure 2: SEC MALS analysis of wildtype TL1A (WT) and the C95S/C135S double cysteine mutant [Cys].
Size exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) was performed to assess the oligomeric state and homogeneity of TL1A WT (red trace) and the C95S/C135S mutant (green trace). Light scattering intensity at 90° is plotted against elution time (minutes). Both samples elute at approximately 25 minutes, consistent with their expected trimeric molecular weight (~62 kDa). The C95S/C135S mutant displays a sharper and more symmetrical peak compared to WT, indicating improved sample homogeneity. Moreover, the lack of an aggregation peak at approximately 21.5 minutes for C95S/C135S indicates improved suitability for structural studies over the WT TL1A.
Structural Biology
Using a combination of broad and literature-informed crystallisation screens, we obtained high-quality crystals of the TL1A mutant. The final structure was solved at 2.2 Å resolution using data collected at Diamond Light Source and belonged to the space group P 1 21 1 (Figure 3). Our structure revealed two trimers per asymmetric unit, offering a unique advantage for future asymmetric binder co-crystallisation. As such, this preliminary work provides a strong foundation for rapidly enabling the solution of TL1A-ligand structures by soaking or co-crystallisation.
Sygnature TL1A [C95S/C135S] Structure:
Data Collection & Refinement Statistics:
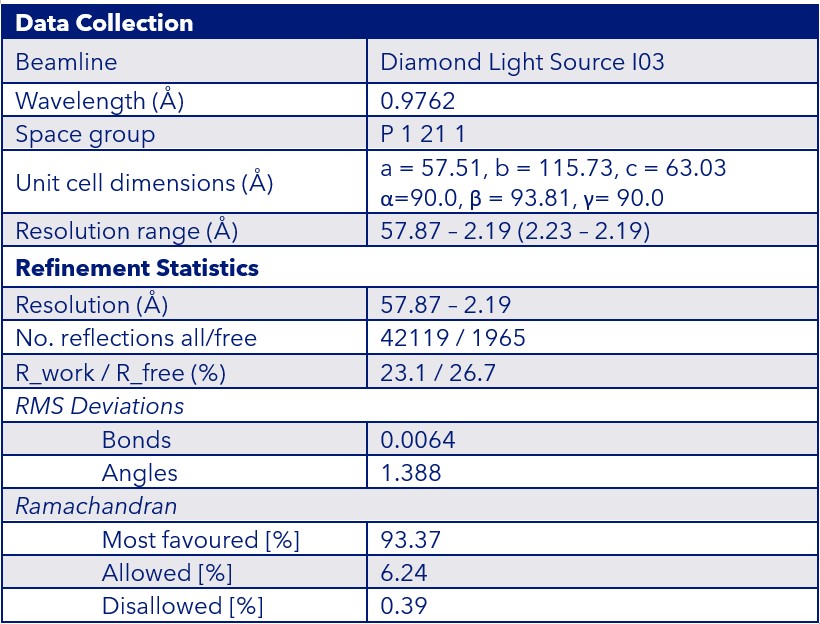
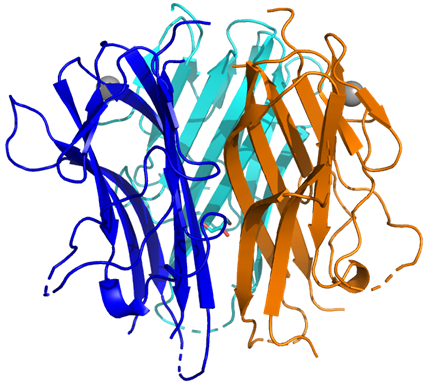
Figure 3: Crystal structure and data collection/refinement statistics for TL1A [C95S/C135S]
The top panel shows the crystal structure of the Sygnature TL1A [C95S/C135S] mutant, with each monomer coloured in blue, cyan, and orange. The bottom panel summarises the key data collection and refinement statistics.
Conclusions
This project showcases our ability to:
- Deliver high quality protein preparations suitable for x-ray crystallography
- Achieve high-resolution structures with drug discovery relevance
- Provide flexible crystallisation platforms for ligand screening
Whether you’re targeting TL1A or another challenging protein, our gene-to-structure workflow and expert team are ready to support your discovery journey.
Get in touch to explore how Sygnature Discovery can accelerate your structural biology goals.
References
- Xu WD, Li R, Huang AF. Role of TL1A in Inflammatory Autoimmune Diseases: A Comprehensive Review. Front Immunol. 2022 Jul 14;13:891328. doi: 10.3389/fimmu.2022.891328.
- Jin T, Guo F, Kim S, Howard A, Zhang YZ. X-ray crystal structure of TNF ligand family member TL1A at 2.1A. Biochem Biophys Res Commun. 2007 Dec 7;364(1):1-6. doi: 10.1016/j.bbrc.2007.09.097.
- Jin T, Kim S, Guo F, Howard A, Zhang YZ. Purification and crystallization of recombinant human TNF-like ligand TL1A. Cytokine. 2007 Nov;40(2):115-22. doi: 10.1016/j.cyto.2007.07.193. Epub 2007 Oct 1.
- Ferdinand JR, Richard AC, Meylan F, Al-Shamkhani A, Siegel RM. Cleavage of TL1A Differentially Regulates Its Effects on Innate and Adaptive Immune Cells. J Immunol. 2018 Feb 15;200(4):1360-1369. doi: 10.4049/jimmunol.1700891. Epub 2018 Jan 15.
- Zhan C, Yan Q, Patskovsky Y, Li Z, Toro R, Meyer A, Cheng H, Brenowitz M, Nathenson SG, Almo SC. Biochemical and structural characterization of the human TL1A ectodomain. Biochemistry. 2009 Aug 18;48(32):7636-45. doi: 10.1021/bi900031w.


